An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.64; 2022 Aug

Global guidelines for breast cancer screening: A systematic review ☆
a Department of Cancer Epidemiology, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China
Mingyang Chen
b Center for Global Health, School of Population Medicine and Public Health, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, China
Youlin Qiao
Fanghui zhao.
Breast cancer screening guidelines could provide valuable tools for clinical decision making by reviewing the available evidence and providing recommendations. Little information is known about how many countries have issued breast cancer screening guidelines and the differences among existing guidelines. We systematically reviewed current guidelines and summarized corresponding recommendations, to provide references for good clinical practice in different countries.
Systematic searches of MEDLINE, EMBASE, Web of Science, and Scopus from inception to March 27th, 2021 were conducted and supplemented by reviewing the guideline development organizations. The quality of screening guidelines was assessed from six domains of the Appraisal of Guidelines for Research and Evaluation Ⅱ (AGREE Ⅱ) instrument by two appraisers. The basic information and recommendations of the issued guidelines were extracted and summarized.
A total of 23 guidelines issued between 2010 and 2021 in 11 countries or regions were identified for further review. The content and quality varied across the guidelines. The average AGREE Ⅱ scores of the guidelines ranged from 33.3% to 87.5%. The highest domain score was "clarity of presentation" while the domain with the lowest score was "applicability". For average-risk women, most of the guidelines recommended mammographic screening for those aged 40–74 years, specifically, those aged 50–69 years were regarded as the optimal age group for screening. Nine of 23 guidelines recommended against an upper age limit for breast cancer screening. Mammography (MAM) was recommended as the primary screening modality for average-risk women by all included guidelines. Most guidelines suggested annual or biennial mammographic screening. Risk factors of breast cancer identified in the guidelines mainly fell within five categories which could be broadly summarized as the personal history of pre-cancerous lesions and/or breast cancer; the family history of breast cancer; the known genetic predisposition of breast cancer; the history of mantle or chest radiation therapy; and dense breasts. For women at higher risk, there was a consensus among most guidelines that annual MAM or annual magnetic resonance imaging (MRI) should be given, and the screening should begin earlier than the average-risk group.
Conclusions
The majority of 23 included international guidelines were issued by developed countries which contained roughly the same but not identical recommendations on breast cancer screening age, methods, and intervals. Most guidelines recommended annual or biennial mammographic screening between 40 and 74 years for average-risk populations and annual MAM or annual MRI starting from a younger age for high-risk populations. Current guidelines varied in quality and increased efforts are needed to improve the methodological quality of guidance documents. Due to lacking clinical practice guidelines tailored to different economic levels, low- and middle-income countries (LMICs) should apply and implement the evidence-based guidelines with higher AGREE Ⅱ scores considering local adaption.
- • This systematic review comprehensively maps the recommendations of the latest international breast screening guidelines, providing valuable tools for clinical decision making in different settings.
- • Most guidelines recommend annual or biennial mammographic screening between 40 and 74 years for the average-risk populations and annual MAM or annual MRI starting from a younger age for the high-risk populations. However, there are indeed discrepancies in screening age, methods, and intervals among countries.
- • High-quality evidence and rigorous methodology are the keys to guidance development, but current guidelines vary in methodological quality.
1. Introduction
In 2021, breast cancer has overtaken lung cancer to be the world's most commonly diagnosed cancer, accounting for the severe burden globally, especially among women [ 1 ]. Screening for breast cancer is an effective measure to detect early-stage disease and improve the survival rate of cancer patients [ [2] , [3] ]. Population-based breast cancer screening programs have been implemented in many developed countries over the last decades, which contributed to reducing the mortality and the advanced cancer rate [ [4] , [5] , [6] ].
Screening guidelines could provide valuable tools for clinical decision making by reviewing the available evidence and providing recommendations. To date, several breast cancer screening guidelines have been issued in many developed countries [ [7] , [8] , [9] ]. However, the recommendations about screening age, methods, and intervals varied from different guidelines due to different institutions, based evidence, and development processes. This may confuse the clinical practice when they are applied to other countries. To our knowledge, it is currently unknown how many countries have issued breast cancer screening guidelines and the differences among these issued guidelines. Additionally, previous systematic reviews of international breast cancer screening guidelines were limited by publication date and screening population and did not systematically review screening recommendations for the population with different breast cancer risks [ [10] , [11] , [12] ].
Accordingly, our study reviewed existing breast cancer screening guidelines and summarized corresponding recommendations, in order to provide references for good clinical practice in different countries.
2. Material and methods
2.1. data sources and searches.
A search strategy was designed for MEDLINE, EMBASE, Web of Science, and Scopus from inception to March 27th, 2021 using variations on the search terms "breast cancer", "screening" and "guidelines/recommendations" ( Appendix A ). We also sought the additional guidelines by searching guideline development organizations, such as Guideline International Network (GIN), World Health Organization (WHO), Cancer Australia, Ministry of Health (MOH) Malaysia, and China Guideline Clearinghouse (CGC). Moreover, we meticulously examined the references of documents obtained above to further access potentially eligible articles.
2.2. Study selection and data extraction
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram is presented in Fig. 1 . Two reviewers (MYC and WHR) independently reviewed the titles and abstracts of the included guidelines. Any discrepancies were resolved by discussion. Finally, both reviewers determined the included guidelines based on the full text. We included guidelines following inclusion criteria: (1) originally published guidelines, consensus, or position papers related to breast cancer screening; (2) the latest versions of the updated guidelines; (3) English or Chinese guidelines; and (4) full text was available. We excluded guidelines if they were: summaries or interpreted versions of guidelines.

Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Two independent reviewers (MYC and WHR) extracted information using a predesigned template. The information extracted included: (1) basic information (countries or regions, publication years, publication organizations, names of guidelines, number of updated versions, and publication years of old versions); (2) screening recommendations for the population at average risk and higher risk (screening age, screening methods, screening intervals, level of evidence, and strength of recommendation).
2.3. Quality assessment
The methodological quality of guidelines was evaluated using the Appraisal of Guidelines for Research and Evaluation Ⅱ (AGREE Ⅱ) instrument. This is a standardized tool for evaluating the methodological framework of guideline development which consists of 23 main items in six domains (scope and purpose, stakeholder involvement, rigour of development, clarity of presentation, applicability, and editorial independence) and two global rating items [ 13 ]. Each item is rated on a seven-point Likert-type scale from one (strongly disagree) to seven (strongly agree) according to the criteria and considerations articulated in the User's Manual. Scores are assigned depending on the completeness and quality of reporting. Scores increase as more criteria are met and considerations are addressed. Domain scores are calculated by summing up all the scores of the individual items in that domain and by scaling the total as a percentage of the maximum possible score for that domain. Two reviewers (MYC and WHR) independently scored each guideline. Evaluation results were compared and discrepancies of more than two points per item were discussed to reach a consensus. According to prior studies, the quality of guidelines was classified as high if the total score was 60% or higher and low if the score was less than 60% [ 14 , 15 ].
A total of 7417 citations were included during the preliminary literature search process, but most were excluded after deleting duplicates and applying the inclusion and exclusion criteria. Of these, 23 guidelines were identified for further review ( Fig. 1 ).
3.1. Guideline characteristics
Table 1 displays the general characteristics of 23 included guidelines that were published between 2010 and 2021 [ [7] , [8] , [9] , [16] , [17] , [18] , [19] , [20] , [21] , [22] , [23] , [24] , [25] , [26] , [27] , [28] , [29] , [30] , [31] , [32] , [33] , [34] , [35] ]. The majority of guidelines (17 of 23) were drawn from developed countries or regions. Guidelines from the United States accounted for the largest proportion, reaching 39.1%. One was developed by WHO, and four in Europe ( Fig. 2 ). 12 of 23 guidelines have been updated.
Characteristics of 23 included guidelines on screening for breast cancer.
Abbreviations: ACOG: American College of Obstetricians and Gynecologists; ACP: American College of Physicians; ACR: American College of Radiology; ACS: American Cancer Society; AWMF: German Association of Scientific Medical Societies; CBR: Brazilian College of Radiology and Diagnostic Imaging; CEWG: Cancer Expert Working Group; CTFPHC: Canadian Task Force on Preventive Health Care; DKG: German Cancer Society; DKH: German Cancer Aid; ECIBC: European Commission Initiative on Breast Cancer; ESMO: European Society for Medical Oncology; EUSOMA: European Society of Breast Cancer Specialists; FEBRASGO: Brazilian Federation of Gynecological and Obstetrical Associations; MOH: Ministry of Health; NCC: National Cancer Centre; NCCN: National Comprehensive Cancer Network; SBI: Society of Breast Imaging; SBM: Brazilian Society for Breast Disease; USPSTF: U.S. Preventive Services Task Force; WHO: World Health Organization.

Geographical distribution of the included breast cancer screening guidelines.
3.2. Quality assessment
The included 23 guidelines were appraised using AGREE II Criteria ( Fig. 3 ). The average AGREE II scores varied from 33.3% to 87.5%. 12 guidelines were scored over 60.0% [ [7] , [8] , [16] , [17] , [23] , [24] , [25] , [27] , [30] , [31] , [32] , [34] ]. Among these, the guideline issued by Canadian Task Force on Preventive Health Care (CTFPHC) [ 27 ] was scored the highest (87.5%), followed by European Commission Initiative on Breast Cancer (ECIBC) [ 25 ], American Cancer Society (ACS) [ 23 ], United States Preventive Services Taskforce (USPSTF) [ 7 ], and WHO [ 16 ]. The highest domain score was "clarity of presentation" (domain 4), with an average score of 81.9%, followed by "scope and purpose" (domain 1). The domain with the lowest score was "applicability" (domain 5) with an average score of 21.3%, followed by "stakeholder involvement" (domain 2).

Quality of the included guidelines for the six domains of the AGREE Ⅱ instrument.
( Abbreviations: ACOG: American College of Obstetricians and Gynecologists; ACP: American College of Physicians; ACR: American College of Radiology; ACS: American Cancer Society; AWMF: German Association of Scientific Medical Societies; CBR: Brazilian College of Radiology and Diagnostic Imaging; CEWG: Cancer Expert Working Group; CTFPHC: Canadian Task Force on Preventive Health Care; ECIBC: European Commission Initiative on Breast Cancer; ESMO: European Society for Medical Oncology; EUSOMA: European Society of Breast Cancer Specialists; MOH: Ministry of Health; NCC: National Cancer Centre; NCCN: National Comprehensive Cancer Network; SBI: Society of Breast Imaging; USPSTF: U.S. Preventive Services Task Force; WHO: World Health Organization).
3.3. Strength of recommendations and quality of evidence
17 of 23 guidelines reported eight applied grading systems. Grading of Recommendations, Assessment, Development and Evaluations (GRADE) was the common system that was applied in six guidelines [ 16 , 23 , 25 , 27 , 32 , 34 ]. Four guidelines used the self-designated grading system [ 8 , 9 , 18 , 30 ]. The details about the strength of recommendations and the quality of evidence varied in different grading systems. The information of evidence and recommendation about the included guidelines is shown in Table 2 .
Grading systems used in the included guidelines.
Abbreviations: ACOG: American College of Obstetricians and Gynecologists; ACS: American Cancer Society; ACR: American College of Radiology; AWMF: German Association of Scientific Medical Societies; CBR: Brazilian College of Radiology and Diagnostic Imaging; CTFPHC: Canadian Task Force on Preventive Health Care; DKG: German Cancer Society; DKH: German Cancer Aid; ECIBC: European Commission Initiative on Breast Cancer; ESMO: European Society for Medical Oncology; EUSOMA: European Society of Breast Cancer Specialists; FEBRASGO: Brazilian Federation of Gynecological and Obstetrical Associations; GPP: Good Practice Points; GRADE: Grading of Recommendations, Assessment, Development and Evaluations; JRGCSG: Japanese Research Group for the Development of Cancer Screening Guidelines; MOH: Ministry of Health; NCC: National Cancer Centre; NCCN: National Comprehensive Cancer Network; OCEBM: Oxford Centre for Evidence-based Medicine; RAM: RAND/UCLA Appropriateness Method; SBM: Brazilian Society for Breast Disease; USPSTF: U.S. Preventive Services Task Force; WHO: World Health Organization.
3.4. The screening recommendations for women at average risk
The detailed information of recommendations for average-risk women is shown in Table 3 , which summarized screening age, screening methods, screening intervals, and other recommended screening methods ( Fig. 4 ).
The screening recommendations in average-risk women in eligible guidelines.
Abbreviations: ABUS: Automated Breast Ultrasonography; ACOG: American College of Obstetricians and Gynecologists; ACP: American College of Physicians; ACR: American College of Radiology; ACS: American Cancer Society; AWMF: German Association of Scientific Medical Societies; BSE: Breast Self-Examination; CBE: Clinical Breast Examination; CBR: The Brazilian College of Radiology and Diagnostic Imaging; CTFPHC: Canadian Task Force on Preventive Health Care; DKG: German Cancer Society; DKH: German Cancer Aid; DBT: Digital Breast Tomosynthesis; ECIBC: European Commission Initiative on Breast Cancer; ESMO: European Society for Medical Oncology; FEBRASGO: Brazilian Federation of Gynecological and Obstetrical Associations; HHUS: Hand-Held Ultrasound; MAM: Mammography; MBI: Molecular Breast Imaging; MOH: Ministry of Health; MRI: Magnetic Resonance Imaging; NCC: National Cancer Centre; NCCN: National Comprehensive Cancer Network; NR: No Recommendation; SBM: The Brazilian Society for Breast Disease; SDM: Shared Decision Making; US: Ultrasound; USPSTF: U.S. Preventive Services Task Force; WHO: World Health Organization.

The main screening recommendations in average-risk women in the eligible guidelines.
( Abbreviations: CBE: Clinical Breast Examination; MAM: Mammography; US: Ultrasound)
3.4.1. Screening age
The majority of guidelines recommended mammographic screening for average-risk individuals aged 40–74 years [ [7] , [8] , [9] , 16 , 17 , 29 , 35 ], and recommended women aged 50–69 years as the optimal age group for screening with strong recommendation [ 8 , 16 , 25 , 28 , 30 , 34 ]. National Comprehensive Cancer Network (NCCN) [ 18 ] and American College of Obstetricians and Gynecologists (ACOG) [ 24 ] suggested starting screening at age 25 by clinical encounter or clinical breast examination (CBE).
Nine of 23 guidelines did not recommend an upper age limit for breast cancer screening [ 8 , 9 , 16 , 18 , 20 , 25 , 27 , 31 , 32 ]. Some guidelines, including American College of Radiology (ACR) [ 21 ], ACR and Society of Breast Imaging (SBI) [ 22 ], and ACS [ 23 ] suggested that the age to end screening should be determined based on the women's health status, for example, stopping screening for women with life expectancy lower than 5–7 years or 10 years. Other guidelines, like USPSTF [ 7 ], American College of Physicians (ACP) [ 17 ], and Brazilian College of Radiology and Diagnostic Imaging (CBR)/Brazilian Society for Breast Disease (SBM)/Brazilian Federation of Gynecological and Obstetrical Associations (FEBRASGO) [ 35 ] did not recommend breast cancer screening for women aged over 75 years unless their life expectancy were higher than 7 years or 10 years. German Association German Cancer Society of Scientific Medical Societies (AWMF)/German Cancer Society (DKG)/German Cancer Aid (DKH) [ 28 ] and MOH of Singapore [ 30 ] recommended stopping screening at age 70.
3.4.2. Screening methods and intervals
Mammography (MAM) was recommended as the primary screening modality for average-risk women by all included guidelines [ [7] , [8] , [9] , [16] , [17] , [18] , [19] , [21] , [22] , [23] , [24] , [25] , [26] , [27] , [28] , [29] , [30] , [31] , [32] , [34] , [35] ]. Most guidelines suggested annual or biennial mammographic screening [ 7 , 16 , 17 , 29 , 31 ]. Three guidelines recommended screening every 1–2 years [ [8] , [23] , [32] ]. Some guidelines agreed that screening intervals should be determined based on age [ 18 , 24 ]. ACS [ 23 ] recommended screening with MAM annually for women aged 40–54 years and every 1–2 years for women aged 55 years or older. ECIBC [ 25 ] recommended screening every 2–3 years for women aged 40–49 years and for women aged 70–74 years. For the priority screening groups (women aged 50–69 years), annual screening was not recommended, and biennial screening is better than triennial screening.
The recommendations of each guideline on CBE and ultrasound (US) were different in detail. NCCN [ 18 ] and ACOG [ 24 ] suggested that CBE should be given every 1–3 years for women aged 25–39 years and annually for women older than 40 years, but ACS [ 23 ] and CTFPHC [ 27 ] did not recommend CBE as a primary screening method. Among the included screening guidelines, only National Cancer Centre (NCC) of China [ 32 ] recommended screening every 1–2 years for women older than 45 years using US alone.
All guidelines did not recommend using breast self-examination (BSE), magnetic resonance imaging (MRI), and computed tomography (CT) to screen for average-risk women because of lacking evidence of benefit.
3.5. The screening recommendations for women at higher risk
Risk factors of breast cancer identified in the guidelines mainly fell within five categories which could be broadly summarized as the personal history of pre-cancerous lesions and/or breast cancer; the family history of breast cancer; the known genetic predisposition of breast cancer; the history of mantle or chest radiation therapy; and dense breasts. For women at higher risk, there was a consensus among most guidelines that annual MAM screening or annual MRI screening should be given and the starting age should be earlier than the average-risk group ( Table 4 ; Fig. 5 ) .
The screening recommendations in high-risk women in eligible guidelines.
Abbreviations: ACR: American College of Radiology; BRCA: Breast cancer gene; BSE: Breast Self-Examination; CBE: Clinical Breast Examination; CBR: Brazilian College of Radiology and Diagnostic Imaging; CEWG: Cancer Expert Working Group; DBT: Digital Breast Tomosynthesis; DM: Digital Mammography; ESMO: European Society for Medical Oncology; EUSOMA: European Society of Breast Cancer Specialists; FEBRASGO: Brazilian Federation of Gynecological and Obstetrical Associations; MAM: mammography; MOH: Ministry of Health; MRI: Magnetic Resonance Imaging; NCC: National Cancer Centre; NCCN: National Comprehensive Cancer Network; NR: No Recommendation; SBI: Society of Breast Imaging; SBM: Brazilian Society for Breast Disease; US: Ultrasound.

The main screening recommendations in high-risk women in the eligible guidelines.
( Abbreviations: BSE: Breast Self Examination; CBE: Clinical Breast Examination; MAM: Mammography; MRI: Magnetic Resonance Imaging; NR: No Recommendation; US: Ultrasound)
3.5.1. Women with the personal history of pre-cancerous lesions and/or breast cancer
For women with biopsy-proven Lobular Carcinoma in Situ (LCIS), Atypical Ductal Hyperplasia (ADH), Ductal Carcinoma in Situ (DCIS), or invasive breast cancer or ovarian cancer, annual MAM or annual MRI was mainly recommended after diagnosis onward [ 18 , 20 , 22 , 31 , 33 , 35 ] . Especially for patients with unilateral invasive breast cancer, close monitoring of the contralateral breast was recommended. NCCN [ 18 ] also recommended breast awareness and clinical encounter every 6–12 months for this group of women. NCC of China [ 32 ] recommended MAM and US as screening methods for women at higher risk of breast cancer.
3.5.2. Women with the family history of breast cancer
For women with a family history suspicious of the inherited predisposition of breast cancer, two guidelines recommended that an annual MAM or annual MRI began 10 years before the age of diagnosis of the youngest-affected relative but not before the age of 30 [ 18 , 33 ]. NCCN [ 18 ] also recommended regular clinical visits every 6–12 months once the women were identified as begin at increased risk of breast cancer.
3.5.3. Women with the known genetic predisposition of breast cancer
Women with breast cancer susceptibility gene 1 (BRCA1) or breast cancer susceptibility gene 2 (BRCA2) mutations, or untested but have first-degree relatives (mothers, sisters, or daughters) who are proven to have BRCA mutations, have a higher risk for breast cancer. Two guidelines recommended that women with gene mutations should start to undertake annual MAM or annual MRI at 25–30 years [ 22 , 30 ]. European Society of Breast Cancer Specialists (EUSOMA) [ 26 ] recommended that annual MRI screening was performed for women carrying BRAC at 25–29 years, and those carrying TP53 at 20 years. MOH of Malaysia [ 31 ] provided age-specific recommendations for women carrying gene mutations, specifically, annual MRI for 30–49 years, annual MAM for 40–69 years, and biennial MAM for 70 years and above. For other recommended screening methods, ACR [ 19 ] recommended MRI as an adjunct to MAM or DBT and recommended US when the patient cannot tolerate MRI. MOH of Singapore [ 30 ] also recommended monthly BSE and 6 monthly CBE.
3.5.4. Women with the history of mantle or chest radiation therapy
For women with a history of mantle or chest radiation therapy that occurred before the age of 30 years or had a cumulative dose of 10 Gy radiation, most guidelines recommended starting regular screening 8 or 10 years after radiation therapy [ [18] , [19] , [20] , 35 ]. Recommended screening strategies included annual MAM (not before age 30), annual MRI (not before age 25), or annual digital mammography (DM) (with or without digital breast tomosynthesis (DBT)). NCCN [ 18 ] also recommended increasing breast awareness or clinical encounters every 6–12 months.
3.5.5. Women with dense breasts
For women with dense breasts, ACR [ 20 ] recommended MRI should be performed annually. NCC of China [ 32 ] recommended screening with MAM and US annually. US (as adjunctive screening tools) was recommended for high-risk women who may be suitable for MRI but can not be accepted for any reason [ 20 ]. Two guidelines [ [22] , [35] ] also recommended US as an adjunctive examination to MAM in asymptomatic women with dense breasts.
4. Discussion
To the best of our knowledge, this study is the largest and most comprehensive systematic review, which identified and compared the latest international breast screening guidelines and recommendations. A total of 23 guidelines issued between 2010 and 2021 in 11 countries or regions were included in this study. The content and quality varied between the guidelines. The average AGREE Ⅱ scores ranged from 33.3% to 87.5%, which is consistent with that reported by Li J et al. [ 12 ]. We found discrepancies between guidelines concerning screening age, methods, and intervals. In general, the majority of guidelines agreed upon annual or biennial MAM for average-risk women aged 40 to 74. Annual MAM or annual MRI should be given and start earlier for women at high risk for breast cancer.
Our study showed that many low- and middle-income countries (LMICs) lacked published clinical practice guidelines for breast cancer screening. Most included guidelines in our study were issued by developed countries, mainly in the United States (9/23) and Europe (4/23). One possible explanation is that high-income countries have accumulated more high-quality evidence for developing guidelines by implementing breast cancer screening programs and related research for a long time [ [4] , [5] , [6] ]. However, although LMICs have a severe breast cancer burden, few tailored guidelines have been issued due to lacking sufficient national evidence about breast cancer screening and the front-line impact of sparse resources to develop guidelines in these areas [ 36 , 37 ]. Additionally, some LMICs guidelines might be published in local languages and were not picked up in our search. We also found that some guidelines issued by LMICs are often based on evidence from high-income countries. The extent to which these guidelines can be applied to the clinical practice of routine screening in LMICs is unknown.
High-quality guidelines are vital to facilitate clinical decision making and to improve health outcomes and health service efficiency. Our findings showed nearly half of the included guidelines were rated as high quality. Most of the guidelines provided a clear description of "scope and purpose" as screening for populations with different breast cancer risks, and screening recommendations were described clearly. For these reasons, the domains "scope and purpose" and "clarity of presentation" received high scores. In contrast, the majority of the guidelines received low scores in the domains of "rigour of development" and "applicability". According to prior studies [ [38] , [39] ], the domain "rigour of development" was the most relevant to the overall quality of the guideline. The main reason was that this domain reflects the evidence collection and synthesis process, as well as the formation and follow-up update of recommendations, which can provide enough information to evaluate whether the guidelines followed the best methodology and developed evidence-based recommendations. Meanwhile, the development process of guidelines is also one of the key reasons causing the variations between the recommendations from different guidance documents. In our study, 17 of 23 guidelines reported using eight different grading systems to evaluate the quality of evidence and strength of recommendations, which somewhat impeded the implementation of the guidelines and caused confusion in clinical practice. The most important purpose of guidelines is to promote their application to real-world medicine practice. Therefore, guideline developers should clearly describe the promotion conditions and hindrance factors in the implementation of recommendations and their improvement strategies, as well as consider the likely resource implications involved. At the same time, the quality of the "applicability" domain also plays a critical role in whether they can be extended to LMICs that might lack indigenous guidelines. Our study showed that the scores of different guidelines varied greatly in the domain "applicability". For example, the guideline issued by MOH of Malaysia [ 31 ] contained a separate section called "implementing the guidelines", which described the types of facilitators and barriers in detail, as well as put forward suggestions to ensure the implementation of the guideline. In contrast, the "ACR Appropriateness Criteria® Breast Cancer Screening" [ 19 ] did not mention facilitators and barriers to its application. Based on the above considerations, we considered the guideline developed by MOH of Malaysia with high "applicability" rather than ACR.
The majority of guidelines recommended mammographic screening for average-risk women aged 40–74 years. 50–69 years were regarded as the optimal age group for screening due to the steep increase of breast cancer beginning around age 50. In 2019, almost 82% of breast cancer was diagnosed among women aged ≥ 50 years in the United States [ 40 ]. Most randomized controlled trials (RCTs) from developed countries also showed that mammographic screening between 50 and 69 years had the greatest benefit in reducing mortality [ [41] , [42] ]. However, due to the disease burden of breast cancer and the allocation of public health resources vary in different countries, a one-size-fits-all approach to screening is considered inapplicable. In several Asian countries, such as Japan and South Korea, the peak age of breast cancer incidence in women mainly ranges from 45 to 69 years old which is more than 10 years earlier than that in Europe and the United States [ 43 , 44 ]. Although some Asian guidelines agreed on beginning screening from the age of 40 or 45 years [ 9 , 30 , 32 ], high-quality evidence from large population-based RCTs is insufficient. In addition, based on several RCTs conducted in Canada, the UK, and Sweden, ECIBC and CTFPHC did not recommend regular screening begin at 40–44 years since the lower absolute benefit and higher overdiagnosis and false positives rate with related biopsies of this age group [ 2 , 25 , 27 , [45] , [46] , [47] ]. Furthermore, nine of 23 guidelines did not recommend an upper age limit. However, some guidelines recommended against regular screening for women older than age 70 or 75 years, as the harm potentially exceeds the benefits if screening is continued after these age groups [ 48 ]. The risk of breast cancer increases with age. Consequently, the decision to stop screening should be individually based on life expectancy or comorbid conditions.
Currently, MAM is widely accepted in developed countries with sufficient evidence to decrease breast cancer mortality among women aged 50–74 years and is recommended as a primary screening method in most screening guidelines [ 49 ]. Due to relatively high cost and the demand for high-quality radiologists, the application of MAM in low resource areas is limited [ 50 ]. Additionally, because higher mammographic density is associated with the masking of breast cancer on a mammogram, the sensitivity of MAM for women with dense breasts is lower than that for women with mainly fatty breasts [ 51 ]. Mammographic density among Asian women is higher than among Western women [ 52 ]. Several Asian studies have shown that US can improve the detection rate of breast cancer for women with dense breasts [ 53 , 54 ]. However, there is limited evidence for US in breast cancer screening to reduce mortality. Accordingly, the guidelines from European and American countries did not recommend US as the primary technique for breast cancer screening in average-risk population, but mainly as a supplemental method to MAM. Among the included guidelines of the present study, only Chinese guidelines recommended US as the primary screening tool. China has carried out a national breast cancer screening program since 2009. The screening tool of the program was changed from CBE to US in 2012, which provided preliminary evidence for the application of US in breast cancer screening in other Asian countries [ 55 , 56 ].
With greater emphasis on more accurate risk management based on patients and more personalized recommendations for diagnosis, treatment, and follow-up, age-oriented screening suggestions have been shifted to risk-based screening recommendations. By accurately identifying women who are above-average risk in the general population, we can provide timely and effective early diagnosis measures. High-risk women identified in the guidelines fell within many categories. The related recommendations for every category of high-risk women were different, which brought some difficulties to the implementation of breast cancer screening for high-risk women in the low resource areas. Thereby, identifying the risk factors of breast cancer by establishing a risk assessment model may be an effective way to prevent breast cancer. Currently, various risk prediction models were developed, such as the Gail model and BOADICEA model, whose application values in different countries are still under evaluation [ 57 , 58 ]. It is reported that China applies risk models as supplementary tools for screening in urban areas [ 59 ].
Few guidelines provided explicit recommendations for the management of women with positive findings except for NCCN [ 18 ] and NCC China [ 32 ]. Improper management of abnormal screening results may compromise the effectiveness of breast cancer screening programs. Doubeni et al. performed the PROSPR multi-model microsimulation study, which showed that the relative risk for the late-stage disease was higher when the time for diagnostic testing was delayed after an abnormal mammogram [ 60 ]. A previous study observed that low-income women and women of ethnic minority (African-American and Asian women) were less likely to have adequate follow-up abnormal breast cancer screening mammograms [ 61 ]. For these reasons, it is necessary to explore different referral and recall standards according to different initial screening results, to make a balance between the anxiety caused by false-positive breast cancer and the benefit of follow-up.
The strengths of this systematic review include its originality and the most comprehensive search strategy. This study was the largest and comprehensive systematic review to map the recommendations of the latest international breast screening guidelines. Furthermore, we systemically summarized the screening recommendations for both average-risk women and high-risk women.
Our study has some limitations. Even though we performed a comprehensive systematic search, we could not find all relevant guidelines. And we also did not include the breast screening program protocols in some countries. Another limitation was that non-English guidelines were not included in this review due to translation restrictions.
5. Conclusions
In summary, this study reviewed and compared the latest international breast screening guidelines for women both at average risk and at higher risk. The majority of guidelines were issued by developed countries, containing roughly the same but not identical recommendations for breast cancer on screening age, methods, and intervals. Most guidelines recommended annual or biennial mammographic screening for average-risk populations aged between 40 and 74 years and early annual MAM or annual MRI for high-risk populations. Current guidelines varied in methodological quality and increased efforts are needed to develop high-quality guidelines to provide more powerful supporting evidence for guidelines users. LMICs lacked published tailored clinical practice guideline. Therefore, we encourage policymakers and clinicians to use the evidence-based guidelines with higher AGREE Ⅱ scores considering local adaption.
Funding source
This work was supported by International Agency for Research on Cancer, France; World Health Organization, Switzerland [grant numbers CRA/SCR/2019/1].
Declaration of competing interest
All authors declare that they have no conflict of interest.
Acknowledgments
We gratefully acknowledge Ms. Huijiao Yan for her linguistic assistance during the revision of this manuscript.
☆ Present address: Department of Cancer Epidemiology, National Cancer Centre/National Clinical Research Centre for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 17 South Pan Jia Yuan Lane, Beijing 100,021, China.
Appendix A. Electronic search strategies
A) medline (via pubmed).
((Breast Neoplasms [MH] OR breast cancer* [tiab] OR breast neoplasm* [tiab] OR breast carcinoma* [tiab] OR breast tumor* [tiab] OR breast tumour* [tiab] OR mammary cancer* [tiab] OR mammary neoplasm* [tiab] OR mammary carcinoma* [tiab] OR mammary tumor* [tiab] OR mammary tumour* [tiab])
("Mass Screening" [Mesh] OR "Early Detection of Cancer" [Mesh] OR screening [tiab] OR early detect*[tiab])
(("Guideline" [Publication Type] OR "Practice Guideline" [Publication Type]) OR ("Guidelines as Topic" [Mesh] OR "Health Planning Guidelines" [Mesh] OR consensus [MeSH]) OR (guideline [Title] OR guidelines [Title] OR "practice guideline" [Title] OR "practice guidelines" [Title] OR "Health Planning Guidelines" [Title] OR Guidance [Title] OR consensus [Title] OR recommendations [Title] OR recommendation [Title] OR manual [Title] OR guidebook [Title] OR guidebooks [Title] OR guide [Title] OR guides [Title] OR handbook [Title] OR handbooks [Title])))
B) EMBASE via embase.com
('breast cancer'/exp OR 'breast tumor'/exp OR 'breast carcinoma*'/exp OR ('breast neoplasm*' OR 'breast tumor*' OR 'breast tumour*' OR 'mammary cancer*' OR 'mammary neoplasm*' OR 'mammary carcinoma*' OR 'mammary tumor*' OR 'mammary tumour*'):ab,ti
('Mass Screening'/exp OR 'early cancer diagnosis'/exp OR "screening":ab, ti OR early detect:ab,ti)
('Practice Guideline'/exp OR 'health care planning'/exp OR consensus/exp OR (guideline OR guidelines OR 'practice guideline' OR 'practice guidelines' OR 'Health Planning Guidelines' OR Guidance OR consensus OR recommendations OR recommendation OR manual OR guidebook OR guidebooks OR guide OR guides OR handbook OR handbooks):ti)
C) Web of Science
TI or AB=("breast cancer*" OR "breast neoplasm*" OR "breast carcinoma*" OR "breast tumor*" OR "breast tumour*" OR "mammary cancer*" OR "mammary neoplasm*" OR "mammary carcinoma*" OR "mammary tumor*" OR "mammary tumour*")
TI or AB=("Mass Screening" OR "Early Detection of Cancer" OR screening OR "early detect*")
TI=(guideline OR guidelines OR "Practice Guideline" OR "practice guidelines" OR consensus OR Guidance OR recommendation OR recommendations OR manual OR guide OR guides OR guidebook OR guidebooks OR handbook OR handbooks)
TITLE-ABS("breast cancer*" OR "breast neoplasm*" OR "breast carcinoma*" OR "breast tumor*" OR "breast tumour*" OR "mammary cancer*" OR "mammary neoplasm*" OR "mammary carcinoma*" OR "mammary tumor*" OR "mammary tumour*")
TITLE-ABS("Mass Screening" OR "Early Detection of Cancer" OR screening OR "early detect*")
TITLE (guideline OR "Practice Guideline" OR consensus OR Guidance OR recommendation OR manual OR guide OR guidebook OR handbook)
- Summary of Recommendations
- USPSTF Assessment of Magnitude of Net Benefit
- Practice Considerations
- Update of Previous USPSTF Recommendation
- Supporting Evidence
- Research Needs and Gaps
- Recommendations of Others
- Article Information
See the “Practice Considerations” section for more information on the patient population to whom this recommendation applies and on screening mammography modalities. USPSTF indicates US Preventive Services Task Force.
ER– indicates estrogen receptor–negative; HER2–, human epidermal growth factor receptor 2–negative; and PR–, progesterone receptor–negative.
eFigure. US Preventive Services Task Force (USPSTF) Grades and Levels of Evidence
- USPSTF Review: Screening for Breast Cancer JAMA US Preventive Services Task Force April 30, 2024 This systematic review to support a 2024 US Preventive Services Task Force Recommendation Statement summarizes published evidence on the benefits and harms of screening for breast cancer in adult females. Jillian T. Henderson, PhD, MPH; Elizabeth M. Webber, MS; Meghan S. Weyrich, MPH; Marykate Miller, MS; Joy Melnikow, MD, MPH
- USPSTF Report: Collaborative Modeling to Compare Breast Cancer Screening Strategies JAMA US Preventive Services Task Force April 30, 2024 This modeling study uses Cancer Intervention and Surveillance Modeling Network models and national data on breast cancer incidence, mammography performance, treatment effects, and other-cause mortality in US women without previous cancer diagnoses to estimate outcomes of various mammography screening strategies. Amy Trentham-Dietz, PhD, MS; Christina Hunter Chapman, MD, MS; Jinani Jayasekera, PhD, MS; Kathryn P. Lowry, MD; Brandy M. Heckman-Stoddard, PhD, MPH; John M. Hampton, MS; Jennifer L. Caswell-Jin, MD; Ronald E. Gangnon, PhD; Ying Lu, PhD, MS; Hui Huang, MS; Sarah Stein, PhD; Liyang Sun, MS; Eugenio J. Gil Quessep, MS; Yuanliang Yang, MS; Yifan Lu, BASc; Juhee Song, PhD; Diego F. Muñoz, PhD; Yisheng Li, PhD, MS; Allison W. Kurian, MD, MSc; Karla Kerlikowske, MD; Ellen S. O’Meara, PhD; Brian L. Sprague, PhD; Anna N. A. Tosteson, ScD; Eric J. Feuer, PhD; Donald Berry, PhD; Sylvia K. Plevritis, PhD; Xuelin Huang, PhD; Harry J. de Koning, MD, PhD; Nicolien T. van Ravesteyn, PhD; Sandra J. Lee, ScD; Oguzhan Alagoz, PhD, MS; Clyde B. Schechter, MD, MA; Natasha K. Stout, PhD; Diana L. Miglioretti, PhD, ScM; Jeanne S. Mandelblatt, MD, MPH
- Toward More Equitable Breast Cancer Outcomes JAMA Editorial April 30, 2024 Joann G. Elmore, MD, MPH; Christoph I. Lee, MD, MS
- Screening for Breast Cancer JAMA JAMA Patient Page April 30, 2024 In this JAMA Patient Page, the US Preventive Services Task Force provides a guide to screening for breast cancer. US Preventive Services Task Force
- When Is It Best to Begin Mammograms, and How Often? JAMA Medical News & Perspectives May 3, 2024 This Medical News story discusses new USPSTF recommendations about the timing of screening mammograms. Rita Rubin, MA
- New Recommendations for Breast Cancer Screening—In Pursuit of Health Equity JAMA Network Open Editorial April 30, 2024 Lydia E. Pace, MD, MPH; Nancy L. Keating, MD, MPH
- USPSTF Breast Cancer Screening Guidelines Do Not Go Far Enough JAMA Oncology Editorial April 30, 2024 Wendie A. Berg, MD, PhD
See More About
Select your interests.
Customize your JAMA Network experience by selecting one or more topics from the list below.
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
Others Also Liked
- Download PDF
- X Facebook More LinkedIn
- CME & MOC
US Preventive Services Task Force. Screening for Breast Cancer : US Preventive Services Task Force Recommendation Statement . JAMA. Published online April 30, 2024. doi:10.1001/jama.2024.5534
Manage citations:
© 2024
- Permissions
Screening for Breast Cancer : US Preventive Services Task Force Recommendation Statement
- Editorial Toward More Equitable Breast Cancer Outcomes Joann G. Elmore, MD, MPH; Christoph I. Lee, MD, MS JAMA
- Editorial New Recommendations for Breast Cancer Screening—In Pursuit of Health Equity Lydia E. Pace, MD, MPH; Nancy L. Keating, MD, MPH JAMA Network Open
- Editorial USPSTF Breast Cancer Screening Guidelines Do Not Go Far Enough Wendie A. Berg, MD, PhD JAMA Oncology
- US Preventive Services Task Force USPSTF Review: Screening for Breast Cancer Jillian T. Henderson, PhD, MPH; Elizabeth M. Webber, MS; Meghan S. Weyrich, MPH; Marykate Miller, MS; Joy Melnikow, MD, MPH JAMA
- US Preventive Services Task Force USPSTF Report: Collaborative Modeling to Compare Breast Cancer Screening Strategies Amy Trentham-Dietz, PhD, MS; Christina Hunter Chapman, MD, MS; Jinani Jayasekera, PhD, MS; Kathryn P. Lowry, MD; Brandy M. Heckman-Stoddard, PhD, MPH; John M. Hampton, MS; Jennifer L. Caswell-Jin, MD; Ronald E. Gangnon, PhD; Ying Lu, PhD, MS; Hui Huang, MS; Sarah Stein, PhD; Liyang Sun, MS; Eugenio J. Gil Quessep, MS; Yuanliang Yang, MS; Yifan Lu, BASc; Juhee Song, PhD; Diego F. Muñoz, PhD; Yisheng Li, PhD, MS; Allison W. Kurian, MD, MSc; Karla Kerlikowske, MD; Ellen S. O’Meara, PhD; Brian L. Sprague, PhD; Anna N. A. Tosteson, ScD; Eric J. Feuer, PhD; Donald Berry, PhD; Sylvia K. Plevritis, PhD; Xuelin Huang, PhD; Harry J. de Koning, MD, PhD; Nicolien T. van Ravesteyn, PhD; Sandra J. Lee, ScD; Oguzhan Alagoz, PhD, MS; Clyde B. Schechter, MD, MA; Natasha K. Stout, PhD; Diana L. Miglioretti, PhD, ScM; Jeanne S. Mandelblatt, MD, MPH JAMA
- JAMA Patient Page Screening for Breast Cancer US Preventive Services Task Force JAMA
- Medical News & Perspectives When Is It Best to Begin Mammograms, and How Often? Rita Rubin, MA JAMA
Importance Among all US women, breast cancer is the second most common cancer and the second most common cause of cancer death. In 2023, an estimated 43 170 women died of breast cancer. Non-Hispanic White women have the highest incidence of breast cancer and non-Hispanic Black women have the highest mortality rate.
Objective The USPSTF commissioned a systematic review to evaluate the comparative effectiveness of different mammography-based breast cancer screening strategies by age to start and stop screening, screening interval, modality, use of supplemental imaging, or personalization of screening for breast cancer on the incidence of and progression to advanced breast cancer, breast cancer morbidity, and breast cancer–specific or all-cause mortality, and collaborative modeling studies to complement the evidence from the review.
Population Cisgender women and all other persons assigned female at birth aged 40 years or older at average risk of breast cancer.
Evidence Assessment The USPSTF concludes with moderate certainty that biennial screening mammography in women aged 40 to 74 years has a moderate net benefit. The USPSTF concludes that the evidence is insufficient to determine the balance of benefits and harms of screening mammography in women 75 years or older and the balance of benefits and harms of supplemental screening for breast cancer with breast ultrasound or magnetic resonance imaging (MRI), regardless of breast density.
Recommendation The USPSTF recommends biennial screening mammography for women aged 40 to 74 years. (B recommendation) The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of screening mammography in women 75 years or older. (I statement) The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of supplemental screening for breast cancer using breast ultrasonography or MRI in women identified to have dense breasts on an otherwise negative screening mammogram. (I statement)
See the Summary of Recommendations figure.
Pathway to Benefit
To achieve the benefit of screening and mitigate disparities in breast cancer mortality by race and ethnicity, it is important that all persons with abnormal screening mammography results receive equitable and appropriate follow-up evaluation and additional testing, inclusive of indicated biopsies, and that all persons diagnosed with breast cancer receive effective treatment.
The US Preventive Services Task Force (USPSTF) makes recommendations about the effectiveness of specific preventive care services for patients without obvious related signs or symptoms to improve the health of people nationwide.
It bases its recommendations on the evidence of both the benefits and harms of the service and an assessment of the balance. The USPSTF does not consider the costs of providing a service in this assessment.
The USPSTF recognizes that clinical decisions involve more considerations than evidence alone. Clinicians should understand the evidence but individualize decision-making to the specific patient or situation. Similarly, the USPSTF notes that policy and coverage decisions involve considerations in addition to the evidence of clinical benefits and harms.
The USPSTF is committed to mitigating the health inequities that prevent many people from fully benefiting from preventive services. Systemic or structural racism results in policies and practices, including health care delivery, that can lead to inequities in health. The USPSTF recognizes that race, ethnicity, and gender are all social rather than biological constructs. However, they are also often important predictors of health risk. The USPSTF is committed to helping reverse the negative impacts of systemic and structural racism, gender-based discrimination, bias, and other sources of health inequities, and their effects on health, throughout its work.
Among all US women, breast cancer is the second most common cancer and the second most common cause of cancer death. In 2023, an estimated 43 170 women died of breast cancer. 1 Non-Hispanic White women have the highest incidence of breast cancer (5-year age-adjusted incidence rate, 136.3 cases per 100 000 women) and non-Hispanic Black women have the second highest incidence rate (5-year age-adjusted incidence rate, 128.3 cases per 100 000 women). 2 Incidence gradually increased among women aged 40 to 49 years from 2000 to 2015 but increased more noticeably from 2015 to 2019, with a 2.0% average annual increase. 3 Despite having a similar or higher self-reported rate of mammography screening, 4 Black women are more likely to be diagnosed with breast cancer beyond stage I than other racial and ethnic groups, are more likely to be diagnosed with triple-negative cancers (ie, estrogen receptor–negative [ER–], progesterone receptor–negative [PR–], and human epidermal growth factor receptor 2–negative [HER2–], which are more aggressive tumors, compared with White women, 5 and are approximately 40% more likely to die of breast cancer compared with White women. 6
The USPSTF concludes with moderate certainty that biennial screening mammography in women aged 40 to 74 years has a moderate net benefit .
The USPSTF concludes that the evidence is insufficient to determine the balance of benefits and harms of screening mammography in women 75 years or older.
The USPSTF concludes that the evidence is insufficient to determine the balance of benefits and harms of supplemental screening for breast cancer with breast ultrasound or MRI, regardless of breast density.
See Table 1 for more information on the USPSTF recommendation rationale and assessment and the eFigure in the Supplement for information on the recommendation grade. See the Figure for a summary of the recommendation for clinicians. For more details on the methods the USPSTF uses to determine the net benefit, see the USPSTF Procedure Manual. 7
These recommendations apply to cisgender women and all other persons assigned female at birth (including transgender men and nonbinary persons) 40 years or older at average risk of breast cancer. This is because the net benefit estimates are driven by sex (ie, female) rather than gender identity, although the studies reviewed for this recommendation generally used the term “women.” These recommendations apply to persons who have factors associated with an increased risk of breast cancer, such as a family history of breast cancer (ie, a first-degree relative with breast cancer) or having dense breasts. They do not apply to persons who have a genetic marker or syndrome associated with a high risk of breast cancer (eg, BRCA1 or BRCA2 genetic variation), a history of high-dose radiation therapy to the chest at a young age, or previous breast cancer or a high-risk breast lesion on previous biopsies. Of note, the USPSTF has a separate recommendation on risk assessment, genetic counseling, and genetic testing for BRCA -related cancer, 8 and family history is a common feature of risk assessment tools that help determine likelihood of BRCA1 or BRCA2 genetic variation.
Both digital mammography and digital breast tomosynthesis (DBT, or “3D mammography”) are effective mammographic screening modalities. DBT must be accompanied by traditional digital mammography or synthetic digital mammography, which is a 2-dimensional image constructed from DBT data 9 , 10 ; hereafter, references to DBT will imply concurrent use with digital mammography or synthetic digital mammography. In general, studies have reported small increases in positive predictive value with DBT compared with digital mammography. Trials reporting on at least 2 consecutive rounds of screening have generally found no statistically significant difference in breast cancer detection or in tumor characteristics (tumor size, histologic grade, or node status) when comparing screening with DBT vs digital mammography. 4
The Breast Cancer Surveillance Consortium (BCSC) is a network of 6 active breast imaging registries and 2 historic registries, providing a large observational database related to breast cancer screening. 11 Collaborative modeling, using inputs from BCSC data, suggests similar benefits and fewer false-positive results with DBT compared with digital mammography. 12 , 13
Available evidence suggests that biennial screening has a more favorable trade-off of benefits vs harms than annual screening. BCSC data showed no difference in detection of cancers stage IIB or higher and cancers with less favorable prognostic characteristics with annual vs biennial screening interval for any age group, 14 and modeling data estimate that biennial screening has a more favorable balance of benefits to harms (eg, life-years gained or breast cancer deaths averted per false-positive result) compared with annual screening. 12
Breast cancer treatment regimens are highly individualized according to each patient’s clinical status, cancer stage, tumor biomarkers, clinical subtype, and personal preferences. 15 Ductal carcinoma in situ (DCIS) is a noninvasive condition with abnormal cells in the breast duct lining with uncertainty regarding its prognostic significance. Consequently, there is clinical variability in the treatment approach when DCIS is identified at screening. It is unknown what proportion of screen-detected DCIS represents overdiagnosis (ie, a lesion that would not have led to health problems in the absence of detection by screening). In general, DCIS treatment, which may include surgery, radiation, and endocrine treatment, is intended to reduce the risk for future invasive breast cancer.
Mortality from breast cancer is highest for Black women, even when accounting for differences in age and stage at diagnosis; mortality is approximately 40% higher for Black women (5-year age-adjusted mortality rate, 27.6 per 100 000 women) compared with White women (5-year age-adjusted mortality rate, 19.7 per 100 000 women). 6 While the underlying causes of this disparity are complex, the National Institute of Minority Health and Disparities has developed a framework that recognizes multiple determinants, including the health care system, the sociocultural and built environments, behavioral factors, and genetic factors, that can contribute to health inequities. 16 Inequities in breast cancer mortality can be examined at each step along the cancer screening, diagnosis, treatment, and survival pathway with these factors in mind. The higher mortality rate for Black women diagnosed with breast cancer in the US aligns with other health inequities that are attributed to the effects of structural racism, which include inequalities in resources, harmful exposures, and access to and delivery of high-quality health care. 17 - 19 Racial and economic residential segregation driven by discriminatory housing policies has been associated with increased exposure to toxic environments such as air pollution, industrial waste, and built environments that do not support health, and stressful life conditions. Residential segregation has also been associated with both an increased risk of triple-negative breast cancer and poorer breast cancer–specific survival in Black women. 20 - 22
Black women have a higher incidence of breast cancer with at least 1 negative molecular marker, and the incidence of triple-negative cancers (ie, ER–, PR–, and HER2–) is twice as high in Black women compared with White women (24.2 vs 12.3 cases per 100 000 women). 5 The higher incidence of negative hormonal receptor status leads to worse outcomes because these subtypes are less readily detected through screening and less responsive to current therapy, 23 and triple-negative cancers are more likely to be aggressive and diagnosed at later stages than other subtypes. It is important to note that observed regional differences in the incidence of hormonal receptor–negative cancer within and between racial groups suggest that environmental factors and social determinants of health, including racism, are largely responsible for the differential risk of developing hormonal receptor–negative cancer. 24 , 25 Although variation in the incidence of cancer subtypes explains some of the differences in breast cancer mortality, racial differences in mortality within subtypes point to barriers to obtaining high-quality health care and disparities in screening follow-up and treatment initiation as contributors. 24
Of note, Black women have a rate of self-reported mammography screening similar to or higher than that for all women (84.5% vs 78%, respectively, in the past 2 years), based on 2020 data. 4 However, benefits from mammography screening require initiation and completion of appropriate and effective follow-up evaluation and treatment. Both screening and guideline-concordant treatment are essential for reducing breast cancer mortality, 26 highlighting the importance of timely and effective treatment at the earliest stage of diagnosis. Delays and inadequacies in the diagnostic and treatment pathway downstream from screening likely contribute to increased mortality compared with women receiving prompt, effective care.
Disparities in follow-up after screening and treatment have been observed for Asian, Black, and Hispanic women. 27 - 36 Adjuvant endocrine therapy reduces the risk of cancer recurrence among individuals with hormonal receptor–positive cancers, but long-term adherence can be difficult. Black women are more likely to discontinue adjuvant endocrine therapy compared with White women, in part due to greater physical (vasomotor, musculoskeletal, or cardiorespiratory) and psychological (distress or despair) symptom burdens. 35 , 36 Improvements in access to effective health care, removal of financial barriers, and use of support services to ensure equitable follow-up after screening and timely and effective treatment of breast cancer have the potential to reduce mortality for individuals experiencing disparities related to racism, rural location, 37 low income, or other factors associated with lower breast cancer survival.
Breast cancer incidence increases with age and peaks among persons aged 70 to 74 years, although rates in persons 75 years or older remain high (453.3 and 409.9 cases per 100 000 women aged 75 to 79 and 80 to 84 years, respectively, compared with 468.2 cases per 100 000 women aged 70 to 74 years), and mortality from breast cancer increases with increasing age. 38 , 39 However, no randomized clinical trials (RCTs) of breast cancer screening included women 75 years or older. 4 Collaborative modeling suggests that screening in women 75 years or older is of benefit, 12 but a trial emulation found no benefit with breast cancer screening in women aged 75 to 84 years. 40 Thus, there is insufficient evidence to recommend for or against screening mammography in women 75 years or older.
In women with dense breasts who have an otherwise normal mammogram result, there is insufficient evidence about the effect of supplemental screening using breast ultrasonography or magnetic resonance imaging (MRI) on health outcomes such as breast cancer morbidity and mortality. Dense breasts are associated with both reduced sensitivity and specificity of mammography and with an increased risk of breast cancer. 41 , 42 However, increased breast density itself is not associated with higher breast cancer mortality among women diagnosed with breast cancer, after adjustment for stage, treatment, method of detection, and other risk factors, according to data from the BCSC. 43
Potential harms of screening mammography include false-positive results, which may lead to psychological harms, 44 additional testing, and invasive follow-up procedures; overdiagnosis and overtreatment of lesions that would not have led to health problems in the absence of detection by screening; and radiation exposure.
Centers for Disease Control and Prevention data show that as of 2015, more than 50% of women 75 years or older reported having a mammogram within the past 2 years. 45 At present, 38 states and the District of Columbia require patient notification of breast density when mammography is performed; in some states, legislation also includes notification language informing women that they should consider adjunctive screening. 46 Starting in September 2024, the US Food and Drug Administration will require mammography centers to notify patients of their breast density, inform them that dense breast tissue increases the risk of breast cancer and makes it harder to detect on a mammogram, and that other imaging tests may help to find cancer. 47
The National Cancer Institute has information on breast cancer screening for health care professionals ( https://www.cancer.gov/types/breast/hp/breast-screening-pdq ) and for patients ( https://www.cancer.gov/types/breast/patient/breast-screening-pdq ).
The Centers for Disease Control and Prevention has information on breast cancer screening ( https://www.cdc.gov/cancer/breast/basic_info/screening.htm ).
The USPSTF has made recommendations about the use of medications to reduce women’s risk for breast cancer 48 as well as risk assessment, genetic counseling, and genetic testing for BRCA1 - or BRCA2 -related cancer. 8
This recommendation updates the 2016 recommendation on breast cancer screening. In 2016, the USPSTF recommended biennial screening mammography for women aged 50 to 74 years and individualizing the decision to undergo screening for women aged 40 to 49 years, based on factors such as individual risk and personal preferences and values. The USPSTF concluded that the evidence was insufficient to assess the benefits and harms of DBT as a primary screening method; the balance of benefits and harms of adjunctive screening for breast cancer using breast ultrasonography, MRI, or DBT in women identified to have dense breasts on an otherwise negative screening mammogram; and the balance of benefits and harms of screening mammography in women 75 years or older. 49 For the current recommendation, the USPSTF recommends biennial screening mammography for women aged 40 to 74 years. The USPSTF again finds that the evidence is insufficient to assess the balance of benefits and harms of supplemental screening for breast cancer using breast ultrasonography or MRI in women identified to have dense breasts on an otherwise negative screening mammogram and the balance of benefits and harms of screening mammography in women 75 years or older. Current evidence suggests that both digital mammography and DBT are effective primary screening modalities.
To update its 2016 recommendation, the USPSTF commissioned a systematic review 4 , 50 on the comparative effectiveness of different mammography-based breast cancer screening strategies by age to start and stop screening, screening interval, modality, use of supplemental imaging, or personalization of screening for breast cancer on the incidence of and progression to advanced breast cancer, breast cancer morbidity, and breast cancer–specific or all-cause mortality. To be included in the review, studies needed to report on detection and stage distribution of screen-detected invasive breast cancer over more than 1 round of screening, to allow assessment for evidence of stage shift (as evidence of potential benefit). Studies that reported only performance characteristics of testing (eg, sensitivity and specificity) or only detection rates were not eligible for inclusion. The review also assessed the harms of different breast cancer screening strategies. 4 Evidence from the trials that established breast cancer screening effectiveness with mammography has not been updated, as there are no new studies that include a group that is not screened. Analyses from prior reviews of that evidence were considered foundational evidence for the current recommendation.
In addition to the systematic evidence review, the USPSTF commissioned collaborative modeling studies from 6 CISNET (Cancer Intervention and Surveillance Modeling Network) modeling teams to provide information about the benefits and harms of breast cancer screening strategies that vary by the ages to begin and end screening, screening modality, and screening interval. 12 In alignment with the USPSTF’s commitment to improve health equity, the USPSTF also commissioned modeling studies from 4 CISNET teams that have developed race-specific breast cancer models for Black women, to provide information about the effectiveness and harms of these different screening strategies in Black women. The USPSTF commissions decision modeling to help inform how best to target or implement a clinical preventive service when empirical evidence supports provision of the service. 51 The modeling studies complement the evidence that the systematic review provides.
Given the documented racial disparities in breast cancer outcomes, in addition to commissioning modeling studies specific to Black women, the evidence review included contextual questions on the drivers behind and approaches to address disparities in health outcomes related to breast cancer, particularly the higher mortality in Black women.
Randomized trials that began enrolling participants more than 30 to 40 years ago have established the effectiveness of screening mammography to reduce breast cancer mortality. A meta-analysis conducted in support of the 2016 USPSTF breast cancer screening recommendation found that screening mammography was associated with relative risk (RR) reductions in breast cancer mortality of 0.88 (95% CI, 0.73-1.00; 9 trials) for women aged 39 to 49 years, 0.86 (95% CI, 0.68-0.97; 7 trials) for women aged 50 to 59 years, 0.67 (95% CI, 0.54-0.83; 5 trials) for women aged 60 to 69 years, and 0.80 (95% CI, 0.51-1.28; 3 trials) for women aged 70 to 74 years, 44 and an updated analysis of 3 Swedish screening trials reported a 15% relative reduction in breast cancer mortality for women aged 40 to 74 years (RR, 0.85 [95% CI, 0.73-0.98]). 52 Only 1 of these trials enrolled a significant proportion of Black women. 53 None of the trials nor the combined meta-analysis demonstrated a difference in all-cause mortality with screening mammography. The current USPSTF review focused on the comparative benefits of different screening strategies.
The USPSTF did not identify any RCTs designed to test the comparative effectiveness of different ages to start or stop screening that reported morbidity, mortality, or quality-of-life outcomes. One trial emulation study (n = 264 274), using a random sample from Medicare claims data, estimated the effect of women stopping screening at age 70 years compared with those who continued annual screening after age 70 years. Based on survival analysis, this study reported that continued screening between the ages of 70 and 74 years was associated with a 22% decrease in the risk of breast cancer mortality, compared with a cessation of screening at age 70 years. While collaborative modeling estimated that, compared with a stopping age of 74, screening biennially starting at age 40 years until age 79 years would lead to 0.8 additional breast cancer deaths averted, the trial emulation study found that there was no difference in the hazard ratio or absolute rates of breast cancer mortality with continued screening vs discontinued screening from ages 75 to 79 years or ages 80 to 84 years. 40
Collaborative modeling data estimated that compared with biennial screening from ages 50 to 74 years, biennial screening starting at age 40 years until 74 years would lead to 1.3 additional breast cancer deaths averted (median, 6.7 vs 8.2, respectively, across 6 models) per 1000 women screened over a lifetime of screening for all women ( Table 2 ; note that the 1.3 deaths averted is the median of the differences in each of 6 models, which is not the same as the difference of the medians noted above and in the table). Models also estimated that screening benefits for Black women are similar for breast cancer mortality reduction and greater for life-years gained and breast cancer deaths averted compared with all women. Thus, biennial screening starting at age 40 years would result in 1.8 additional breast cancer deaths averted (median, 9.2 deaths averted for screening from ages 50 to 74 vs 10.7 deaths averted, across 4 models) per 1000 women screened for Black women ( Table 2 ; note that the 1.8 deaths averted is the median of the differences in each of 4 models, which is not the same as the difference of the medians noted above and in the table). 12 Epidemiologic data has shown that the incidence rate of invasive breast cancer for 40- to 49-year-old women has increased an average of 2.0% annually between 2015 and 2019, a higher rate than in previous years. 3 These factors led the USPSTF to conclude that screening mammography in women aged 40 to 49 years has a moderate benefit by reducing the number of breast cancer deaths.
The USPSTF did not identify any randomized trials directly comparing annual vs biennial screening that reported morbidity, mortality, or quality-of-life outcomes. One trial (n = 14 765) conducted in Finland during the years 1985 to 1995 assigned participants aged 40 to 49 years to annual or triennial screening invitations based on birth year (even birth year: annual; odd birth year: triennial) and reported similar mortality from incident breast cancer and for all-cause mortality between the 2 groups, with follow-up to age 52 years. 54
A nonrandomized study using BCSC data (n = 15 440) compared the tumor characteristics of cancers detected following annual vs biennial screening intervals. 14 The relative risk of being diagnosed with a stage IIB or higher cancer and cancer with less favorable characteristics was not statistically different for biennially vs annually screened women in any of the age categories. The risk of a stage IIB or higher cancer diagnosis and of having a tumor with less favorable prognostic characteristics was higher for premenopausal women screened biennially vs annually (RR, 1.28 [95% CI, 1.01-1.63] and RR, 1.11 [95% CI, 1.00-1.22], respectively). However, this study did not conduct formal tests for interaction in the subgroup comparisons and did not adjust for multiple comparisons.
One RCT (n = 76 022) conducted between 1989 and 1996 randomized individuals to annual or triennial screening and reported on breast cancer incidence. The number of screen-detected cancers was higher in the annual screening study group (RR, 1.64 [95% CI, 1.28-2.09]). However, the total number of cancers diagnosed either clinically or with screening was similar after 3 years of screening. Cancers occurring in the annual screening group (including clinically diagnosed cancers) did not differ by prognostic features such as tumor size, node positivity status, or histologic grade compared with those in the triennial screening group. 55
Collaborative modeling estimated that biennial screening results in greater incremental life-years gained and mortality reduction per mammogram and has a more favorable balance of benefits to harms for all women and for Black women, compared with annual screening. While modeling suggests that screening Black women annually and screening other women biennially would reduce the disparity in breast cancer mortality, 12 , 13 trial or observational evidence is lacking that screening any group of women annually compared with biennial screening improves mortality from breast cancer. 4
The USPSTF did not identify any RCTs or observational studies that compared screening with DBT vs digital mammography and reported morbidity, mortality, or quality-of-life outcomes.
Three RCTs 56 - 58 and 1 nonrandomized study 59 compared detection of invasive cancer over 2 rounds of screening with DBT vs digital mammography. These trials screened all participants with the same screening modality at the second screening round—digital mammography in 2 trials and the nonrandomized study and DBT in 1 trial. Stage shift or differences in tumor characteristics across screening rounds could offer indirect evidence of potential screening benefit. The trials found no statistically significant difference in detection at the second screening round (pooled RR, 0.87 [95% CI, 0.73-1.05]; 3 trials [n = 105 064]). 4 , 50 The nonrandomized study (n = 92 404) found higher detection at round 1 for the group screened with DBT and higher detection at round 2 for the group screened with digital mammography at both rounds. There were no statistically significant differences in tumor diameter, histologic grade, and node status at the first or second round of screening in any of these studies.
Collaborative modeling data estimated that the benefits of DBT are similar to the estimated benefits of digital mammography (eg, approximately 5 to 6 more life-years gained per 1000 women screened). 12 , 13
The USPSTF found no studies of supplemental screening with MRI or ultrasonography, or studies of personalized (eg, risk-based) screening strategies, that reported on morbidity or mortality or on cancer detection and characteristics over multiple rounds of screening. 4 , 50 Collaborative modeling studies did not investigate the effects of screening with MRI or ultrasonography. Modeling generally estimated that the benefits of screening mammography would be greater for persons at modestly increased risk (eg, the risk of breast cancer associated with a first-degree family history of breast cancer). 12 , 13
For this recommendation, the USPSTF also reviewed the harms of screening for breast cancer and whether the harms varied by screening strategy. Potential harms of screening for breast cancer include false-positive and false-negative results, need for additional imaging and biopsy, overdiagnosis, and radiation exposure.
The most common harm is a false-positive result, which can lead to psychological harms such as anxiety or breast cancer–specific worry, 44 as well as additional testing and invasive follow-up procedures without the potential for benefit. Collaborative modeling data estimated that a strategy of screening biennially from ages 40 to 74 years would result in 1376 false-positive results per 1000 women screened over a lifetime of screening ( Table 2 ). 12 , 13
Overdiagnosis occurs when breast cancer that would never have become a threat to a person’s health, or even apparent, during their lifetime is found due to screening. It is not possible to directly observe for any individual person whether they have or do not have an overdiagnosed tumor; it is only possible to indirectly estimate the frequency of overdiagnosis that may occur across a screened population. Estimates of the percentage of cancers diagnosed in a study that represent overdiagnosed cancers from RCTs that had comparable groups at baseline, had adequate follow-up, and did not provide screening to the control group at the end of the trial range from approximately 11% to 19%. 4 , 50 Collaborative modeling data estimate that a strategy of screening biennially from ages 40 to 74 years would lead to 14 overdiagnosed cases of breast cancer per 1000 persons screened over the lifetime of screening ( Table 2 ), although with a very wide range of estimates (4 to 37 cases) across models. 12 , 13
One trial emulation (n = 264 274) compared discontinuation of mammography screening at age 70 years or older with continued annual screening beyond this age. 40 Overall, the 8-year cumulative risk of a breast cancer diagnosis was higher for the continued annual screening strategy after age 70 years (5.5% overall; 5.3% in women aged 70-74 years; 5.8% in women aged 75-84 years) compared with the stop screening strategy (3.9% overall; same proportion for both age groups). Fewer cancers were diagnosed under the stop screening strategy (ages 70-84 years), resulting in a lower risk of undergoing follow-up and treatment. For women aged 75 to 84 years, additional diagnoses did not contribute to a difference in the risk of breast cancer mortality, likely due to competing causes of death, raising the possibility that the additionally diagnosed cancers represent overdiagnosis.
Collaborative modeling data estimated that lowering the age to start screening to 40 years from 50 years would result in about a 60% increase in false-positive results, and 2 additional overdiagnosed cases of breast cancer (range, 0 to 4) per 1000 women over a lifetime of screening ( Table 2 ). 12 , 13
Rates of interval cancers (cancer diagnosis occurring between screening) reported in screening studies reflect a combination of cancers that were missed during previous screening examinations (false-negative results) and incident cancers emerging between screening rounds. Evidence from studies comparing various intervals and reporting on the effect of screening interval on the rate of interval cancers is mixed. One RCT comparing annual vs triennial screening reported that the rate of interval cancers was significantly lower in the annual invitation group (1.84 cases per 1000 women initially screened) than in the triennial invitation group (2.70 cases per 1000 women initially screened) (RR, 0.68 [95% CI, 0.50-0.92]), 55 while a quasi-randomized study, also comparing annual vs triennial screening, found no difference in the number of interval cancers between the 2 groups. 54
Based on 2 studies, false-positive results were more likely to occur with annual screening compared with longer intervals between screening. 60 , 61 One of these studies, using data from the BCSC, reported that biennial screening led to a 5% absolute decrease in the 10-year cumulative false-positive biopsy rate compared with annual screening, whether screening was conducted with DBT or digital mammography. 60 Collaborative modeling estimated that annual screening results in more false-positive results and breast cancer overdiagnosis. For example, a strategy of screening annually from ages 40 to 74 years would result in about 50% more false-positive results and 50% more overdiagnosed cases of breast cancer compared with biennial screening for all women and a similar increase in false-positive results and a somewhat smaller increase in overdiagnosed cases for Black women. 12 , 13
Three RCTs did not show statistically significant differences in the risk of interval cancer following screening with DBT or digital mammography (pooled RR, 0.87 [95% CI, 0.64-1.17]; 3 trials [n = 130 196]). 4 , 50 Five nonrandomized studies generally support the RCT findings. Three of the nonrandomized studies found no significant difference in the rate of interval cancers diagnosed following screening with DBT or digital mammography, 59 , 62 , 63 while 1 study found a slight increased risk with DBT screening 64 and 1 study found an unadjusted decreased risk with DBT screening. 65
A pooled analysis of 3 RCTs (n = 105 244) comparing screening with DBT vs digital mammography did not find a difference in false-positive results at the second round of screening. 4 , 50 A nonrandomized study using BCSC data reported that the estimated cumulative probability of having at least 1 false-positive result over 10 years of screening was generally lower with DBT screening compared with digital mammography screening (annual screening: 10-year cumulative probability of a false-positive result was 49.6% with DBT and 56.3% with digital mammography; biennial screening: 10-year cumulative probability of a false-positive result was 35.7% for DBT and 38.1% for digital mammography). The risk of having a biopsy over 10 years of screening was slightly lower when comparing annual screening with DBT vs digital mammography but did not differ between DBT and digital mammography for biennial screening (annual screening: 10-year cumulative probability of a false-positive biopsy was 11.2% with DBT and 11.7% with digital mammography; biennial screening: 10-year cumulative probability of a false-positive biopsy was 6.6% for DBT and 6.7% for digital mammography). When results were stratified by breast density, the difference in false-positive result probability with DBT vs digital mammography was largest for women with nondense breasts and was not significantly different among women with extremely dense breasts. 60 Collaborative modeling, using inputs from BCSC data, estimated that screening women aged 40 to 74 years with DBT would result in 167 fewer false-positive results (range, 166-169) per 1000 persons screened, compared with digital mammography. 12 , 13
In the 3 RCTs cited above, rates of DCIS detected did not differ between persons screened with DBT and digital mammography. 56 - 58
Screening with DBT includes evaluation of 2-dimensional images, generated either with digital mammography or using a DBT scan to produce a synthetic digital mammography image. 9 , 10 Studies using DBT with digital mammography screening reported radiation exposure approximately 2 times higher compared with the digital mammography–only control group. 56 , 58 , 66 Differences in radiation exposure were smaller in studies using DBT/synthetic digital mammography compared with digital mammography. 67 , 68
The DENSE RCT, which compared invitation to screening with digital mammography plus MRI compared with digital mammography alone in participants aged 50 to 75 years with extremely dense breasts and a negative mammogram result, reported a significantly lower rate of invasive interval cancers—2.2 cases per 1000 women invited to screening with digital mammography plus MRI, compared with 4.7 cases per 1000 women invited to screening with digital mammography only (RR, 0.47 [95% CI, 0.29-0.77]). 69
In that trial, the rate of recall among participants who underwent additional imaging with MRI was 94.9 per 1000 screens, the false-positive rate was 79.8 per 1000 women screened, and the rate of biopsy was 62.7 per 1000 women screened. 70 In a nonrandomized study using US insurance claims data, individuals who had an MRI compared with those receiving only a mammogram were more likely in the subsequent 6 months to have additional cascade events related to extramammary findings (adjusted difference between groups, 19.6 per 100 women screened [95% CI, 8.6-30.7]), mostly additional health care visits. 71
In an RCT comparing screening with digital mammography plus ultrasonography vs digital mammography alone conducted in persons aged 40 to 49 years and not specifically among persons with dense breasts, the interval cancer rates reported were not statistically significantly different between the 2 groups (RR, 0.58 [95% CI, 0.31-1.08]) 72 ; similarly, in a nonrandomized study comparing digital mammography plus ultrasonography vs digital mammography alone using BCSC data, there was no difference in interval cancers (adjusted RR, 0.67 [95% CI, 0.33-1.37]), 73 although in both studies the confidence intervals were wide for this uncommon outcome. In the BCSC analysis, the rates of referral to biopsy and false-positive biopsy recommendations were twice as high and short interval follow-up was 3 times higher for the group screened with ultrasonography. 73
A draft version of this recommendation statement was posted for public comment on the USPSTF website from May 9, 2023, to June 6, 2023. The USPSTF received many comments on the draft recommendation and appreciates all the thoughtful views and perspectives that were shared. Many comments agreed with the draft recommendation. Several comments suggested that there should be no upper age limit for breast cancer screening or that an upper age should be based on life expectancy. In response, the USPSTF notes that no trials of breast cancer screening enrolled women 75 years or older and an emulated trial showed no benefit to screening women aged 75 to 79 or 80 to 84. Some comments suggested that breast cancer screening should start prior to age 40 years, either for all women or for women who are at increased risk of breast cancer. Relatedly, some comments expressed that risk-based screening should be recommended. In response, the USPSTF would like to reiterate that no trials of breast cancer screening enrolled women younger than 39 years. Additionally, the USPSTF found no evidence on the benefits or harms of individualized breast cancer screening based on risk factors. Several randomized trials of risk-based screening are underway (eg, the WISDOM trial) that may provide valuable information regarding this question.
Several comments expressed that breast cancer screening should be recommended annually. In response, the USPSTF would like to reiterate that it did not identify any randomized trials directly comparing annual vs biennial screening. Two trials conducted in the 1980s to 1990s reported no difference in breast cancer mortality or breast cancer features such as tumor size, node positivity status, or histologic grade when comparing annual vs triennial screening. The USPSTF considers both the benefits and harms of different screening intervals and notes that the modeling studies commissioned to support this recommendation found that biennial screening results in greater life-years gained and mortality reduction per mammogram and has a more favorable balance of benefits to harms compared with annual screening.
Many comments requested that the USPSTF recommend supplemental screening with MRI or ultrasound for women with dense breasts. Some comments expressed that this would improve health outcomes, while other comments requested this recommendation so that supplemental screening would be covered by insurance. In response, the USPSTF wants to restate that it found insufficient evidence on the effects of supplemental screening on health outcomes. No studies of supplemental screening reported on health outcomes or on the incidence of and progression to advanced breast cancer over more than 1 round of screening. The USPSTF wants all women to be able to get the care they need and would like to clarify that the I statement is not a recommendation for or against supplemental screening in women with dense breasts. It fundamentally means that there is insufficient evidence to assess the balance of benefits and harms, or to recommend for or against supplemental screening, and that women should talk with their clinicians about what is best given their individual circumstances. The USPSTF is also calling for more research to help close this important evidence gap.
Some comments requested clarification of the patient population included in this recommendation, particularly as it relates to women with a family history of breast cancer or those with a genetic predisposition to increased breast cancer risk. In response, the USPSTF clarified that this recommendation applies to women with a family history of breast cancer but not those who have a genetic marker or syndrome or chest radiation exposure at a young age associated with a high risk of breast cancer. The USPSTF also clarified that it has an existing recommendation on risk assessment, genetic counseling, and genetic testing for BRCA -related cancer.
Some comments expressed that racial and ethnic disparities in breast cancer outcomes, especially in Black women, need to be comprehensively addressed. Related comments expressed that the higher breast cancer mortality that Black women experience is primarily related to their not receiving follow-up evaluation and treatment of the same timeliness and quality as White women, and that starting screening at age 40 years will not remedy this inequity. The USPSTF agrees that mitigating disparities in breast cancer mortality is crucial and highlights these disparities in the Disparities in Breast Cancer Outcomes and Implementation Considerations section of this recommendation statement. The USPSTF also agrees that improvements across the entire spectrum of breast cancer care are needed to reduce mortality for individuals experiencing disparities associated with lower breast cancer survival. For this recommendation, current evidence shows that screening for breast cancer starting at age 40 years will be of significant benefit to Black women. The USPSTF is also calling for more research to understand the underlying causes of why Black women are more likely to be diagnosed with breast cancers that have biomarker patterns that confer greater risk for poor health outcomes, to understand the causes of and ways to mitigate the higher mortality from breast cancer that Black women experience.
Some comments disagreed with the USPSTF B recommendation for screening women between the ages of 40 and 49 years, questioned the evidence to support this, or expressed that the current recommendation downplays the harms of screening. In response, the USPSTF has clarified that it uses modeling to complement trial and observational evidence when there is empirical (ie, trial) evidence of the benefit of a preventive service on health outcomes, as there is for breast cancer screening. Decision modeling can assist the USPSTF in assessing the magnitude of the benefits and harms of different screening strategies. The USPSTF carefully weighs both the benefits and harms of a preventive service as it makes its recommendations and currently concludes, as it has in the past, that the benefits of breast cancer screening outweigh the harms for women between the ages of 40 and 49 years. The most recent epidemiologic data reviewed by the USPSTF show greater incidence of breast cancer at younger ages, and decision modeling shows a greater magnitude of benefit for screening women between the ages of 40 and 49 years. The USPSTF considered both these lines of evidence as it issued its current B recommendation for biennial screening mammography for women aged 40 to 74 years.
Last, in response to comments, the USPSTF added the breast cancer screening recommendations from the American College of Radiology to the Recommendations of Others section.
See Table 3 for research needs and gaps related to screening for breast cancer.
The American Cancer Society recommends that women with an average risk of breast cancer should undergo regular screening mammography starting at age 45 years. It suggests that women aged 45 to 54 years should be screened annually, that women 55 years or older should transition to biennial screening or have the opportunity to continue screening annually, that women should have the opportunity to begin annual screening between the ages of 40 and 44 years, and that women should continue screening mammography as long as their overall health is good and they have a life expectancy of 10 years or longer. 74
The American College of Obstetricians and Gynecologists recommends that women at average risk of breast cancer should be offered screening mammography starting at age 40 years, using shared decision-making, and if they have not initiated screening in their 40s, they should begin screening mammography by no later than age 50 years. It recommends that women at average risk of breast cancer should have screening mammography every 1 or 2 years and should continue screening mammography until at least age 75 years. Beyond age 75 years, the decision to discontinue screening mammography should be based on shared decision-making informed by the woman’s health status and longevity. 75
The American College of Radiology and the Society of Breast Imaging recommend annual screening mammography beginning at age 40 years for women at average risk. They recommend that screening should continue past age 74 years, without an upper age limit, unless severe comorbidities limit life expectancy. 76 The American College of Radiology also recommends breast cancer risk assessment by age 25 years for all individuals. 77
The American Academy of Family Physicians supports the 2016 USPSTF recommendation on screening for breast cancer. 78
Accepted for Publication: March 24, 2024.
Published Online: April 30, 2024. doi:10.1001/jama.2024.5534
Corresponding Author: Wanda K. Nicholson, MD, MPH, MBA, Milken Institute of Public Health, George Washington University, 950 New Hampshire Ave NW #2, Washington, DC 20052 ( [email protected] ).
The US Preventive Services Task Force (USPSTF) Members: Wanda K. Nicholson, MD, MPH, MBA; Michael Silverstein, MD, MPH; John B. Wong, MD; Michael J. Barry, MD; David Chelmow, MD; Tumaini Rucker Coker, MD, MBA; Esa M. Davis, MD, MPH; Carlos Roberto Jaén, MD, PhD, MS; Marie Krousel-Wood, MD, MSPH; Sei Lee, MD, MAS; Li Li, MD, PhD, MPH; Carol M. Mangione, MD, MSPH; Goutham Rao, MD; John M. Ruiz, PhD; James J. Stevermer, MD, MSPH; Joel Tsevat, MD, MPH; Sandra Millon Underwood, PhD, RN; Sarah Wiehe, MD, MPH.
Affiliations of The US Preventive Services Task Force (USPSTF) Members: George Washington University, Washington, DC (Nicholson); Brown University, Providence, Rhode Island (Silverstein); Tufts University School of Medicine, Boston, Massachusetts (Wong); Harvard Medical School, Boston, Massachusetts (Barry); Virginia Commonwealth University, Richmond (Chelmow); University of Washington, Seattle (Coker); University of Maryland School of Medicine, Baltimore (Davis); The University of Texas Health Science Center, San Antonio (Jaén, Tsevat); Tulane University, New Orleans, Louisiana (Krousel-Wood); University of California, San Francisco (Lee); University of Virginia, Charlottesville (Li); University of California, Los Angeles (Mangione); Case Western Reserve University, Cleveland, Ohio (Rao); University of Arizona, Tucson (Ruiz); University of Missouri, Columbia (Stevermer); University of Wisconsin, Milwaukee (Underwood); Indiana University, Bloomington (Wiehe).
Author Contributions: Dr Nicholson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The USPSTF members contributed equally to the recommendation statement.
Conflict of Interest Disclosures: Authors followed the policy regarding conflicts of interest described at https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/conflict-interest-disclosures . All members of the USPSTF receive travel reimbursement and an honorarium for participating in USPSTF meetings. Dr Wong reported delivering numerous unpaid talks on the 2009 USPSTF breast cancer screening recommendation; serving as a paid statistical reviewer for review of the USPSTF breast cancer screening models for the Annals of Internal Medicine in 2016 and of Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer and other cancer models submitted to the Annals of Internal Medicine ; serving as an unpaid National Cancer Institute chair of a group (2 others) that performed an external evaluation of the CISNET Program to assess its past productivity and adherence to its mission in 2018 (offered payment but never received); and serving as an unpaid member of the National Cancer Institute–convened Multi-cancer Early Detection (MCED) Diagnostic Pathways Working Group. Dr Chelmow reported serving as chair of the American College of Obstetricians and Gynecologists Practice Advisory Committee; in this role, he was involved in the development of practice guidelines related to breast cancer screening. Dr Barry reported receiving grants from Healthwise, a nonprofit, outside the submitted work. Dr Lee reported receiving grants from the National Institute on Aging (K24AG066998, R01AG079982) outside the submitted work. No other disclosures were reported.
Funding/Support: The USPSTF is an independent, voluntary body. The US Congress mandates that the Agency for Healthcare Research and Quality (AHRQ) support the operations of the USPSTF.
Role of the Funder/Sponsor: AHRQ staff assisted in the following: development and review of the research plan, commission of the systematic evidence review from an Evidence-based Practice Center, coordination of expert review and public comment of the draft evidence report and draft recommendation statement, and the writing and preparation of the final recommendation statement and its submission for publication. AHRQ staff had no role in the approval of the final recommendation statement or the decision to submit for publication.
Disclaimer: Recommendations made by the USPSTF are independent of the US government. They should not be construed as an official position of AHRQ or the US Department of Health and Human Services.
Additional Contributions: We thank Howard Tracer, MD (AHRQ), who contributed to the writing of the manuscript, and Lisa Nicolella, MA (AHRQ), who assisted with coordination and editing.
Additional Information: Published by JAMA®—Journal of the American Medical Association under arrangement with the Agency for Healthcare Research and Quality (AHRQ). ©2024 AMA and United States Government, as represented by the Secretary of the Department of Health and Human Services (HHS), by assignment from the members of the United States Preventive Services Task Force (USPSTF). All rights reserved.
- Register for email alerts with links to free full-text articles
- Access PDFs of free articles
- Manage your interests
- Save searches and receive search alerts

Recommendations
Public comments and nominations, about the uspstf.
- Recommendation Topics
- Recommendation: Breast Cancer: Screening
Final Recommendation Statement
Breast cancer: screening, april 30, 2024.
Recommendations made by the USPSTF are independent of the U.S. government. They should not be construed as an official position of the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services.
Screening Saves Lives from Breast Cancer: Finalized Guidance
The Task Force now recommends that all women get screened every other year starting at age 40. This final recommendation also urgently calls for research in key areas.
Explore this page to learn more about the latest Task Force final recommendation on screening for breast cancer.
Dr. Wanda Nicholson shares key information about the recommendation.
Frequently asked questions.
The Task Force recommends that all women get screened for breast cancer every other year, starting at age 40 and continuing through age 74, to reduce their risk of dying from this disease. This is a B grade .
In this final recommendation statement, we are also urgently calling for more research that will allow us to build on our existing guidance and help all women live longer and healthier lives. Specifically, we need to know how best to address health disparities across screening and treatment experienced by Black, Hispanic, Latina, Asian, Pacific Islander, Native American, and Alaska Native women. We also need studies on what more should be done for women with dense breasts, and we need evidence on the benefits and harms of screening in older women. These are I statements .
While we have consistently recognized the value of mammography, the latest science makes it clear that we can save even more lives from breast cancer. Previously, we recommended that women in their 40s make an individual decision with their clinician on when they should start screening, taking into account their health history, preferences, and how they value the different potential benefits and harms.
The Task Force now recommends that all women start getting screened for breast cancer every other year starting at age 40. Basically, it’s a shift from recommending women start screening between the ages of 40 and 50 to recommending that all women start getting screened when they turn 40.
Nearly half of all women have dense breasts, which increases their risk for breast cancer and means that mammograms may not work as well for them. Women are generally told that they have dense breasts after they’ve had a mammogram. These women deserve to know whether and how additional screening might help them stay healthy. Unfortunately, there is not yet enough evidence for the Task Force to recommend for or against additional screening with breast ultrasound or MRI. We are urgently calling for more research on whether and how additional screening might help women with dense breasts find cancers earlier.
It is important to note that all women, including those with dense breasts, should be screened starting at age 40. While we call for more research, these women should talk to their clinicians about their options for follow-up testing so that they can get the care that’s right for them.
Black women are 40 percent more likely to die from breast cancer than White women and too often get aggressive cancers at young ages. Ensuring Black women start screening at 40 is an important first step, yet it is not enough to improve these inequities. It’s important that patients receive equitable and appropriate follow-up after screening and effective treatment of breast cancer. We are urgently calling for more evidence to better understand whether Black women could potentially be helped by different screening strategies.
Get the Facts
- April 30, 2024 | Associated Press Mammograms should start at 40 to address rising breast cancer rates at younger ages, panel says April 30, 2024 | USPSTF Task Force Issues Final Recommendation Statement on Screening for Breast Cancer April 30, 2024 | JAAPA AAPA talks with USPSTF about its new breast cancer screening recommendation
Media Contact
- Email: [email protected]
- Phone: (301) 951-9203
- Visit the Newsroom
Recommendation Summary
Pathway to benefit.
To achieve the benefit of screening and mitigate disparities in breast cancer mortality by race and ethnicity, it is important that all persons with abnormal screening mammography results receive equitable and appropriate follow-up evaluation and additional testing, inclusive of indicated biopsies, and that all persons diagnosed with breast cancer receive effective treatment.
Clinician Summary
The USPSTF recognizes that clinical decisions involve more considerations than evidence alone. Clinicians should understand the evidence but individualize decision-making to the specific patient or situation.
- View the Clinician Summary in PDF
Additional Information
- Supporting Evidence and Research Taxonomy
- Related Resources & Tools
- Final Evidence Review (April 30, 2024)
- Final Modeling Report (April 30, 2024)
- Modeling Study (April 30, 2024)
- Evidence Summary (April 30, 2024)
- Evidence Gaps Research Taxonomy Table (April 30, 2024)
- Final Research Plan (May 06, 2021)
- Breast Cancer: Information for Professionals - For Providers New Resource for Clinicians and Patients
- Q&A with Journal of the American Academy of PAs - For Providers New Resource for Clinicians and Patients
- Let's Talk About It: Screening for Breast Cancer - For Providers New Resource for Clinicians and Patients
- Breast Cancer: Information for Patients New Resource for Clinicians and Patients
- JAMA Podcast: Screening for Breast Cancer New Resource for Clinicians and Patients
- What Is Breast Cancer Screening? New Resource for Clinicians and Patients
Recommendation Information
Full recommendation:.
The US Preventive Services Task Force (USPSTF) makes recommendations about the effectiveness of specific preventive care services for patients without obvious related signs or symptoms to improve the health of people nationwide.
It bases its recommendations on the evidence of both the benefits and harms of the service and an assessment of the balance. The USPSTF does not consider the costs of providing a service in this assessment.
The USPSTF recognizes that clinical decisions involve more considerations than evidence alone. Clinicians should understand the evidence but individualize decision-making to the specific patient or situation. Similarly, the USPSTF notes that policy and coverage decisions involve considerations in addition to the evidence of clinical benefits and harms.
The USPSTF is committed to mitigating the health inequities that prevent many people from fully benefiting from preventive services. Systemic or structural racism results in policies and practices, including health care delivery, that can lead to inequities in health. The USPSTF recognizes that race, ethnicity, and gender are all social rather than biological constructs. However, they are also often important predictors of health risk. The USPSTF is committed to helping reverse the negative impacts of systemic and structural racism, gender-based discrimination, bias, and other sources of health inequities, and their effects on health, throughout its work.
Among all US women, breast cancer is the second most common cancer and the second most common cause of cancer death. In 2023, an estimated 43,170 women died of breast cancer. 1 Non-Hispanic White women have the highest incidence of breast cancer (5-year age-adjusted incidence rate, 136.3 cases per 100,000 women) and non-Hispanic Black women have the second highest incidence rate (5-year age-adjusted incidence rate, 128.3 cases per 100,000 women). 2 Incidence gradually increased among women aged 40 to 49 years from 2000 to 2015 but increased more noticeably from 2015 to 2019, with a 2.0% average annual increase. 3 Despite having a similar or higher self-reported rate of mammography screening, 4 Black women are more likely to be diagnosed with breast cancer beyond stage I than other racial and ethnic groups, are more likely to be diagnosed with triple-negative cancers (ie, estrogen receptor–negative [ER–], progesterone receptor–negative [PR–], and human epidermal growth factor receptor 2–negative [HER2–]), which are more aggressive tumors, compared with White women, 5 and are approximately 40% more likely to die of breast cancer compared with White women. 6
The USPSTF concludes with moderate certainty that biennial screening mammography in women aged 40 to 74 years has a moderate net benefit .
The USPSTF concludes that the evidence is insufficient to determine the balance of benefits and harms of screening mammography in women 75 years or older.
The USPSTF concludes that the evidence is insufficient to determine the balance of benefits and harms of supplemental screening for breast cancer with breast ultrasound or MRI, regardless of breast density.
See Table 1 for more information on the USPSTF recommendation rationale and assessment. For more details on the methods the USPSTF uses to determine the net benefit, see the USPSTF Procedure Manual. 7
Patient Population Under Consideration
These recommendations apply to cisgender women and all other persons assigned female at birth (including transgender men and nonbinary persons) 40 years or older at average risk of breast cancer. This is because the net benefit estimates are driven by sex (ie, female) rather than gender identity, although the studies reviewed for this recommendation generally used the term “women.” These recommendations apply to persons who have factors associated with an increased risk of breast cancer, such as a family history of breast cancer (ie, a first-degree relative with breast cancer) or having dense breasts. They do not apply to persons who have a genetic marker or syndrome associated with a high risk of breast cancer (eg, BRCA1 or BRCA2 genetic variation), a history of high-dose radiation therapy to the chest at a young age, or previous breast cancer or a high-risk breast lesion on previous biopsies. Of note, the USPSTF has a separate recommendation on risk assessment, genetic counseling, and genetic testing for BRCA-related cancer, 8 and family history is a common feature of risk assessment tools that help determine likelihood of BRCA1 or BRCA2 genetic variation.
Screening Tests
Both digital mammography and digital breast tomosynthesis (DBT, or “3D mammography”) are effective mammographic screening modalities. DBT must be accompanied by traditional digital mammography or synthetic digital mammography, which is a 2-dimensional image constructed from DBT data; 9 , 10 hereafter, references to DBT will imply concurrent use with digital mammography or synthetic digital mammography. In general, studies have reported small increases in positive predictive value with DBT compared with digital mammography. Trials reporting on at least 2 consecutive rounds of screening have generally found no statistically significant difference in breast cancer detection or in tumor characteristics (tumor size, histologic grade, or node status) when comparing screening with DBT vs digital mammography. 4
The Breast Cancer Surveillance Consortium (BCSC) is a network of 6 active breast imaging registries and 2 historic registries, providing a large observational database related to breast cancer screening. 11 Collaborative modeling, using inputs from BCSC data, suggests similar benefits and fewer false-positive results with DBT compared with digital mammography. 12 , 13
Screening Interval
Available evidence suggests that biennial screening has a more favorable trade-off of benefits vs harms than annual screening. BCSC data showed no difference in detection of cancers stage IIB or higher and cancers with less favorable prognostic characteristics with annual vs biennial screening interval for any age group, 14 and modeling data estimate that biennial screening has a more favorable balance of benefits to harms (eg, life-years gained or breast cancer deaths averted per false-positive result) compared with annual screening. 12
Treatment or Intervention
Breast cancer treatment regimens are highly individualized according to each patient’s clinical status, cancer stage, tumor biomarkers, clinical subtype, and personal preferences. 15 Ductal carcinoma in situ (DCIS) is a noninvasive condition with abnormal cells in the breast duct lining with uncertainty regarding its prognostic significance. Consequently, there is clinical variability in the treatment approach when DCIS is identified at screening. It is unknown what proportion of screen-detected DCIS represents overdiagnosis (ie, a lesion that would not have led to health problems in the absence of detection by screening). In general, DCIS treatment, which may include surgery, radiation, and endocrine treatment, is intended to reduce the risk for future invasive breast cancer.
Disparities in Breast Cancer Outcomes and Implementation Considerations
Mortality from breast cancer is highest for Black women, even when accounting for differences in age and stage at diagnosis; mortality is approximately 40% higher for Black women (5-year age-adjusted mortality rate, 27.6 per 100,000 women) compared with White women (5-year age-adjusted mortality rate, 19.7 per 100,000 women). 6 While the underlying causes of this disparity are complex, the National Institute of Minority Health and Disparities has developed a framework that recognizes multiple determinants, including the health care system, the sociocultural and built environments, behavioral factors, and genetic factors, that can contribute to health inequities. 16 Inequities in breast cancer mortality can be examined at each step along the cancer screening, diagnosis, treatment, and survival pathway with these factors in mind. The higher mortality rate for Black women diagnosed with breast cancer in the US aligns with other health inequities that are attributed to the effects of structural racism, which include inequalities in resources, harmful exposures, and access to and delivery of high-quality health care. 17-19 Racial and economic residential segregation driven by discriminatory housing policies has been associated with increased exposure to toxic environments such as air pollution, industrial waste, and built environments that do not support health, and stressful life conditions. Residential segregation has also been associated with both an increased risk of triple-negative breast cancer and poorer breast cancer–specific survival in Black women. 20-22
Black women have a higher incidence of breast cancer with at least 1 negative molecular marker, and the incidence of triple-negative cancers (ie, ER–, PR–, and HER2–) is twice as high in Black women compared with White women (24.2 vs 12.3 cases per 100,000 women). 5 The higher incidence of negative hormonal receptor status leads to worse outcomes because these subtypes are less readily detected through screening and less responsive to current therapy, 23 and triple-negative cancers are more likely to be aggressive and diagnosed at later stages than other subtypes. It is important to note that observed regional differences in the incidence of hormonal receptor–negative cancer within and between racial groups suggest that environmental factors and social determinants of health, including racism, are largely responsible for the differential risk of developing hormonal receptor–negative cancer. 24 , 25 Although variation in the incidence of cancer subtypes explains some of the differences in breast cancer mortality, racial differences in mortality within subtypes point to barriers to obtaining high-quality health care and disparities in screening follow-up and treatment initiation as contributors. 24
Of note, Black women have a rate of self-reported mammography screening similar to or higher than that for all women (84.5% vs 78%, respectively, in the past 2 years), based on 2020 data. 4 However, benefits from mammography screening require initiation and completion of appropriate and effective follow-up evaluation and treatment. Both screening and guideline-concordant treatment are essential for reducing breast cancer mortality, 26 highlighting the importance of timely and effective treatment at the earliest stage of diagnosis. Delays and inadequacies in the diagnostic and treatment pathway downstream from screening likely contribute to increased mortality compared with women receiving prompt, effective care.
Disparities in follow-up after screening and treatment have been observed for Asian, Black, and Hispanic women. 27-36 Adjuvant endocrine therapy reduces the risk of cancer recurrence among individuals with hormonal receptor–positive cancers, but long-term adherence can be difficult. Black women are more likely to discontinue adjuvant endocrine therapy compared with White women, in part due to greater physical (vasomotor, musculoskeletal, or cardiorespiratory) and psychological (distress or despair) symptom burdens. 35 , 36 Improvements in access to effective health care, removal of financial barriers, and use of support services to ensure equitable follow-up after screening and timely and effective treatment of breast cancer have the potential to reduce mortality for individuals experiencing disparities related to racism, rural location, 37 low income, or other factors associated with lower breast cancer survival.
Suggestions for Practice Regarding the I Statement
Potential preventable burden.
Breast cancer incidence increases with age and peaks among persons aged 70 to 74 years, although rates in persons 75 years or older remain high (453.3 and 409.9 cases per 100,000 women aged 75 to 79 and 80 to 84 years, respectively, compared with 468.2 cases per 100,000 women aged 70 to 74 years), and mortality from breast cancer increases with increasing age. 38 , 39 However, no randomized clinical trials (RCTs) of breast cancer screening included women 75 years or older. 4 Collaborative modeling suggests that screening in women 75 years or older is of benefit, 12 but a trial emulation found no benefit with breast cancer screening in women aged 75 to 84 years. 40 Thus, there is insufficient evidence to recommend for or against screening mammography in women 75 years or older.
In women with dense breasts who have an otherwise normal mammogram result, there is insufficient evidence about the effect of supplemental screening using breast ultrasonography or magnetic resonance imaging (MRI) on health outcomes such as breast cancer morbidity and mortality. Dense breasts are associated with both reduced sensitivity and specificity of mammography and with an increased risk of breast cancer. 41 , 42 However, increased breast density itself is not associated with higher breast cancer mortality among women diagnosed with breast cancer, after adjustment for stage, treatment, method of detection, and other risk factors, according to data from the BCSC. 43
Potential Harms
Potential harms of screening mammography include false-positive results, which may lead to psychological harms, 44 additional testing, and invasive follow-up procedures; overdiagnosis and overtreatment of lesions that would not have led to health problems in the absence of detection by screening; and radiation exposure.
Current Practice
Centers for Disease Control and Prevention data show that as of 2015, more than 50% of women 75 years or older reported having a mammogram within the past 2 years. 45 At present, 38 states and the District of Columbia require patient notification of breast density when mammography is performed; in some states, legislation also includes notification language informing women that they should consider adjunctive screening. 46 Starting in September 2024, the US Food and Drug Administration will require mammography centers to notify patients of their breast density, inform them that dense breast tissue increases the risk of breast cancer and makes it harder to detect on a mammogram, and that other imaging tests may help to find cancer. 47
Additional Tools and Resources
The National Cancer Institute has information on breast cancer screening for health care professionals ( https://www.cancer.gov/types/breast/hp/breast-screening-pdq ) and for patients ( https://www.cancer.gov/types/breast/patient/breast-screening-pdq ).
The Centers for Disease Control and Prevention has information on breast cancer screening ( https://www.cdc.gov/cancer/breast/basic_info/screening.htm ).
Other Related USPSTF Recommendations
The USPSTF has made recommendations about the use of medications to reduce women’s risk for breast cancer 48 as well as risk assessment, genetic counseling, and genetic testing for BRCA1 - or BRCA2 -related cancer. 8
This recommendation updates the 2016 recommendation on breast cancer screening. In 2016, the USPSTF recommended biennial screening mammography for women aged 50 to 74 years and individualizing the decision to undergo screening for women aged 40 to 49 years, based on factors such as individual risk and personal preferences and values. The USPSTF concluded that the evidence was insufficient to assess the benefits and harms of DBT as a primary screening method; the balance of benefits and harms of adjunctive screening for breast cancer using breast ultrasonography, MRI, or DBT in women identified to have dense breasts on an otherwise negative screening mammogram; and the balance of benefits and harms of screening mammography in women 75 years or older. 49 For the current recommendation, the USPSTF recommends biennial screening mammography for women aged 40 to 74 years. The USPSTF again finds that the evidence is insufficient to assess the balance of benefits and harms of supplemental screening for breast cancer using breast ultrasonography or MRI in women identified to have dense breasts on an otherwise negative screening mammogram and the balance of benefits and harms of screening mammography in women 75 years or older. Current evidence suggests that both digital mammography and DBT are effective primary screening modalities.
Scope of Review
To update its 2016 recommendation, the USPSTF commissioned a systematic review 4 , 50 on the comparative effectiveness of different mammography-based breast cancer screening strategies by age to start and stop screening, screening interval, modality, use of supplemental imaging, or personalization of screening for breast cancer on the incidence of and progression to advanced breast cancer, breast cancer morbidity, and breast cancer–specific or all-cause mortality. To be included in the review, studies needed to report on detection and stage distribution of screen-detected invasive breast cancer over more than 1 round of screening, to allow assessment for evidence of stage shift (as evidence of potential benefit). Studies that reported only performance characteristics of testing (eg, sensitivity and specificity) or only detection rates were not eligible for inclusion. The review also assessed the harms of different breast cancer screening strategies. 4 Evidence from the trials that established breast cancer screening effectiveness with mammography has not been updated, as there are no new studies that include a group that is not screened. Analyses from prior reviews of that evidence were considered foundational evidence for the current recommendation.
In addition to the systematic evidence review, the USPSTF commissioned collaborative modeling studies from 6 CISNET (Cancer Intervention and Surveillance Modeling Network) modeling teams to provide information about the benefits and harms of breast cancer screening strategies that vary by the ages to begin and end screening, screening modality, and screening interval. 12 In alignment with the USPSTF’s commitment to improve health equity, the USPSTF also commissioned modeling studies from 4 CISNET teams that have developed race-specific breast cancer models for Black women, to provide information about the effectiveness and harms of these different screening strategies in Black women. The USPSTF commissions decision modeling to help inform how best to target or implement a clinical preventive service when empirical evidence supports provision of the service. 51 The modeling studies complement the evidence that the systematic review provides.
Given the documented racial disparities in breast cancer outcomes, in addition to commissioning modeling studies specific to Black women, the evidence review included contextual questions on the drivers behind and approaches to address disparities in health outcomes related to breast cancer, particularly the higher mortality in Black women.
Benefits and Comparative Benefits of Early Detection and Treatment
Randomized trials that began enrolling participants more than 30 to 40 years ago have established the effectiveness of screening mammography to reduce breast cancer mortality. A meta-analysis conducted in support of the 2016 USPSTF breast cancer screening recommendation found that screening mammography was associated with relative risk (RR) reductions in breast cancer mortality of 0.88 (95% CI, 0.73-1.00; 9 trials) for women aged 39 to 49 years, 0.86 (95% CI, 0.68-0.97; 7 trials) for women aged 50 to 59 years, 0.67 (95% CI, 0.54-0.83; 5 trials) for women aged 60 to 69 years, and 0.80 (95% CI, 0.51-1.28; 3 trials) for women aged 70 to 74 years, 44 and an updated analysis of 3 Swedish screening trials reported a 15% relative reduction in breast cancer mortality for women aged 40 to 74 years (RR, 0.85 [95% CI, 0.73-0.98]). 52 Only 1 of these trials enrolled a significant proportion of Black women. 53 None of the trials nor the combined meta-analysis demonstrated a difference in all-cause mortality with screening mammography. The current USPSTF review focused on the comparative benefits of different screening strategies.
Age to Start or Stop Screening
The USPSTF did not identify any RCTs designed to test the comparative effectiveness of different ages to start or stop screening that reported morbidity, mortality, or quality-of-life outcomes. One trial emulation study (n = 264,274), using a random sample from Medicare claims data, estimated the effect of women stopping screening at age 70 years compared with those who continued annual screening after age 70 years. Based on survival analysis, this study reported that continued screening between the ages of 70 and 74 years was associated with a 22% decrease in the risk of breast cancer mortality, compared with a cessation of screening at age 70 years. While collaborative modeling estimated that, compared with a stopping age of 74, screening biennially starting at age 40 years until age 79 years would lead to 0.8 additional breast cancer deaths averted, the trial emulation study found that there was no difference in the hazard ratio or absolute rates of breast cancer mortality with continued screening vs discontinued screening from ages 75 to 79 years or ages 80 to 84 years. 40
Collaborative modeling data estimated that compared with biennial screening from ages 50 to 74 years, biennial screening starting at age 40 years until 74 years would lead to 1.3 additional breast cancer deaths averted (median, 6.7 vs 8.2, respectively, across 6 models) per 1000 women screened over a lifetime of screening for all women ( Table 2 ; note that the 1.3 deaths averted is the median of the differences in each of 6 models, which is not the same as the difference of the medians noted above and in the table). Models also estimated that screening benefits for Black women are similar for breast cancer mortality reduction and greater for life-years gained and breast cancer deaths averted compared with all women. Thus, biennial screening starting at age 40 years would result in 1.8 additional breast cancer deaths averted (median, 9.2 deaths averted for screening from ages 50 to 74 vs 10.7 deaths averted, across 4 models) per 1000 women screened for Black women ( Table 2 ; note that the 1.8 deaths averted is the median of the differences in each of 4 models, which is not the same as the difference of the medians noted above and in the table). 12 Epidemiologic data has shown that the incidence rate of invasive breast cancer for 40- to 49-year-old women has increased an average of 2.0% annually between 2015 and 2019, a higher rate than in previous years. 3 These factors led the USPSTF to conclude that screening mammography in women aged 40 to 49 years has a moderate benefit by reducing the number of breast cancer deaths.
The USPSTF did not identify any randomized trials directly comparing annual vs biennial screening that reported morbidity, mortality, or quality-of-life outcomes. One trial (n = 14,765) conducted in Finland during the years 1985 to 1995 assigned participants aged 40 to 49 years to annual or triennial screening invitations based on birth year (even birth year: annual; odd birth year: triennial) and reported similar mortality from incident breast cancer and for all-cause mortality between the 2 groups, with follow-up to age 52 years. 54
A nonrandomized study using BCSC data (n = 15,440) compared the tumor characteristics of cancers detected following annual vs biennial screening intervals. 14 The relative risk of being diagnosed with a stage IIB or higher cancer and cancer with less favorable characteristics was not statistically different for biennially vs annually screened women in any of the age categories. The risk of a stage IIB or higher cancer diagnosis and of having a tumor with less favorable prognostic characteristics was higher for premenopausal women screened biennially vs annually (RR, 1.28 [95% CI, 1.01-1.63] and RR, 1.11 [95% CI, 1.00-1.22], respectively). However, this study did not conduct formal tests for interaction in the subgroup comparisons and did not adjust for multiple comparisons.
One RCT (n = 76,022) conducted between 1989 and 1996 randomized individuals to annual or triennial screening and reported on breast cancer incidence. The number of screen-detected cancers was higher in the annual screening study group (RR, 1.64 [95% CI, 1.28-2.09]). However, the total number of cancers diagnosed either clinically or with screening was similar after 3 years of screening. Cancers occurring in the annual screening group (including clinically diagnosed cancers) did not differ by prognostic features such as tumor size, node positivity status, or histologic grade compared with those in the triennial screening group. 55
Collaborative modeling estimated that biennial screening results in greater incremental life-years gained and mortality reduction per mammogram and has a more favorable balance of benefits to harms for all women and for Black women, compared with annual screening. While modeling suggests that screening Black women annually and screening other women biennially would reduce the disparity in breast cancer mortality, 12 , 13 trial or observational evidence is lacking that screening any group of women annually compared with biennial screening improves mortality from breast cancer. 4
DBT vs Digital Mammography
The USPSTF did not identify any RCTs or observational studies that compared screening with DBT vs digital mammography and reported morbidity, mortality, or quality-of-life outcomes.
Three RCTs 56-58 and 1 nonrandomized study 59 compared detection of invasive cancer over 2 rounds of screening with DBT vs digital mammography. These trials screened all participants with the same screening modality at the second screening round—digital mammography in 2 trials and the nonrandomized study and DBT in 1 trial. Stage shift or differences in tumor characteristics across screening rounds could offer indirect evidence of potential screening benefit. The trials found no statistically significant difference in detection at the second screening round (pooled RR, 0.87 [95% CI, 0.73-1.05]; 3 trials [n = 105,064]). 4 , 50 The nonrandomized study (n = 92,404) found higher detection at round 1 for the group screened with DBT and higher detection at round 2 for the group screened with digital mammography at both rounds. There were no statistically significant differences in tumor diameter, histologic grade, and node status at the first or second round of screening in any of these studies.
Collaborative modeling data estimated that the benefits of DBT are similar to the estimated benefits of digital mammography (eg, approximately 5 to 6 more life-years gained per 1000 women screened). 12 , 13
Supplemental Screening With MRI or Ultrasonography, or Personalized Screening
The USPSTF found no studies of supplemental screening with MRI or ultrasonography, or studies of personalized (eg, risk-based) screening strategies, that reported on morbidity or mortality or on cancer detection and characteristics over multiple rounds of screening. 4 , 50 Collaborative modeling studies did not investigate the effects of screening with MRI or ultrasonography. Modeling generally estimated that the benefits of screening mammography would be greater for persons at modestly increased risk (eg, the risk of breast cancer associated with a first-degree family history of breast cancer). 12 , 13
Harms of Screening
For this recommendation, the USPSTF also reviewed the harms of screening for breast cancer and whether the harms varied by screening strategy. Potential harms of screening for breast cancer include false-positive and false-negative results, need for additional imaging and biopsy, overdiagnosis, and radiation exposure.
The most common harm is a false-positive result, which can lead to psychological harms such as anxiety or breast cancer–specific worry, 44 as well as additional testing and invasive follow-up procedures without the potential for benefit. Collaborative modeling data estimated that a strategy of screening biennially from ages 40 to 74 years would result in 1376 false-positive results per 1000 women screened over a lifetime of screening ( Table 2 ). 12 , 13
Overdiagnosis occurs when breast cancer that would never have become a threat to a person’s health, or even apparent, during their lifetime is found due to screening. It is not possible to directly observe for any individual person whether they have or do not have an overdiagnosed tumor; it is only possible to indirectly estimate the frequency of overdiagnosis that may occur across a screened population. Estimates of the percentage of cancers diagnosed in a study that represent overdiagnosed cancers from RCTs that had comparable groups at baseline, had adequate follow-up, and did not provide screening to the control group at the end of the trial range from approximately 11% to 19%. 4 , 50 Collaborative modeling data estimate that a strategy of screening biennially from ages 40 to 74 years would lead to 14 overdiagnosed cases of breast cancer per 1000 persons screened over the lifetime of screening ( Table 2 ), although with a very wide range of estimates (4 to 37 cases) across models. 12 , 13
One trial emulation (n = 264,274) compared discontinuation of mammography screening at age 70 years or older with continued annual screening beyond this age.40 Overall, the 8-year cumulative risk of a breast cancer diagnosis was higher for the continued annual screening strategy after age 70 years (5.5% overall; 5.3% in women aged 70-74 years; 5.8% in women aged 75-84 years) compared with the stop screening strategy (3.9% overall; same proportion for both age groups). Fewer cancers were diagnosed under the stop screening strategy (ages 70-84 years), resulting in a lower risk of undergoing follow-up and treatment. For women aged 75 to 84 years, additional diagnoses did not contribute to a difference in the risk of breast cancer mortality, likely due to competing causes of death, raising the possibility that the additionally diagnosed cancers represent overdiagnosis.
Collaborative modeling data estimated that lowering the age to start screening to 40 years from 50 years would result in about a 60% increase in false-positive results, and 2 additional overdiagnosed cases of breast cancer (range, 0 to 4) per 1000 women over a lifetime of screening ( Table 2 ). 12 , 13
Rates of interval cancers (cancer diagnosis occurring between screening) reported in screening studies reflect a combination of cancers that were missed during previous screening examinations (false-negative results) and incident cancers emerging between screening rounds. Evidence from studies comparing various intervals and reporting on the effect of screening interval on the rate of interval cancers is mixed. One RCT comparing annual vs triennial screening reported that the rate of interval cancers was significantly lower in the annual invitation group (1.84 cases per 1000 women initially screened) than in the triennial invitation group (2.70 cases per 1000 women initially screened) (RR, 0.68 [95% CI, 0.50-0.92]), 55 while a quasi-randomized study, also comparing annual vs triennial screening, found no difference in the number of interval cancers between the 2 groups. 54
Based on 2 studies, false-positive results were more likely to occur with annual screening compared with longer intervals between screening. 60 , 61 One of these studies, using data from the BCSC, reported that biennial screening led to a 5% absolute decrease in the 10-year cumulative false-positive biopsy rate compared with annual screening, whether screening was conducted with DBT or digital mammography. 60 Collaborative modeling estimated that annual screening results in more false-positive results and breast cancer overdiagnosis. For example, a strategy of screening annually from ages 40 to 74 years would result in about 50% more false-positive results and 50% more overdiagnosed cases of breast cancer compared with biennial screening for all women and a similar increase in false-positive results and a somewhat smaller increase in overdiagnosed cases for Black women. 12 , 13
Three RCTs did not show statistically significant differences in the risk of interval cancer following screening with DBT or digital mammography (pooled RR, 0.87 [95% CI, 0.64-1.17]; 3 trials [n = 130,196]). 4 , 50 Five nonrandomized studies generally support the RCT findings. Three of the nonrandomized studies found no significant difference in the rate of interval cancers diagnosed following screening with DBT or digital mammography, 59 , 62 , 63 while 1 study found a slight increased risk with DBT screening 64 and 1 study found an unadjusted decreased risk with DBT screening. 65
A pooled analysis of 3 RCTs (n = 105,244) comparing screening with DBT vs digital mammography did not find a difference in false-positive results at the second round of screening. 4 , 50 A nonrandomized study using BCSC data reported that the estimated cumulative probability of having at least 1 false-positive result over 10 years of screening was generally lower with DBT screening compared with digital mammography screening (annual screening: 10-year cumulative probability of a false-positive result was 49.6% with DBT and 56.3% with digital mammography; biennial screening: 10-year cumulative probability of a false-positive result was 35.7% for DBT and 38.1% for digital mammography). The risk of having a biopsy over 10 years of screening was slightly lower when comparing annual screening with DBT vs digital mammography but did not differ between DBT and digital mammography for biennial screening (annual screening: 10-year cumulative probability of a false-positive biopsy was 11.2% with DBT and 11.7% with digital mammography; biennial screening: 10-year cumulative probability of a false-positive biopsy was 6.6% for DBT and 6.7% for digital mammography). When results were stratified by breast density, the difference in false-positive result probability with DBT vs digital mammography was largest for women with nondense breasts and was not significantly different among women with extremely dense breasts. 60 Collaborative modeling, using inputs from BCSC data, estimated that screening women aged 40 to 74 years with DBT would result in 167 fewer false-positive results (range, 166-169) per 1000 persons screened, compared with digital mammography. 12 , 13
In the 3 RCTs cited above, rates of DCIS detected did not differ between persons screened with DBT and digital mammography. 56-58
Screening with DBT includes evaluation of 2-dimensional images, generated either with digital mammography or using a DBT scan to produce a synthetic digital mammography image. 9 , 10 Studies using DBT with digital mammography screening reported radiation exposure approximately 2 times higher compared with the digital mammography–only control group. 56 , 58 , 66 Differences in radiation exposure were smaller in studies using DBT/synthetic digital mammography compared with digital mammography. 67 , 68
Supplemental Screening With Ultrasonography or MRI
The DENSE RCT, which compared invitation to screening with digital mammography plus MRI compared with digital mammography alone in participants aged 50 to 75 years with extremely dense breasts and a negative mammogram result, reported a significantly lower rate of invasive interval cancers—2.2 cases per 1000 women invited to screening with digital mammography plus MRI, compared with 4.7 cases per 1000 women invited to screening with digital mammography only (RR, 0.47 [95% CI, 0.29-0.77]). 69
In that trial, the rate of recall among participants who underwent additional imaging with MRI was 94.9 per 1000 screens, the false-positive rate was 79.8 per 1000 women screened, and the rate of biopsy was 62.7 per 1000 women screened. 70 In a nonrandomized study using US insurance claims data, individuals who had an MRI compared with those receiving only a mammogram were more likely in the subsequent 6 months to have additional cascade events related to extramammary findings (adjusted difference between groups, 19.6 per 100 women screened [95% CI, 8.6-30.7]), mostly additional health care visits. 71
In an RCT comparing screening with digital mammography plus ultrasonography vs digital mammography alone conducted in persons aged 40 to 49 years and not specifically among persons with dense breasts, the interval cancer rates reported were not statistically significantly different between the 2 groups (RR, 0.58 [95% CI, 0.31-1.08]); 72 similarly, in a nonrandomized study comparing digital mammography plus ultrasonography vs digital mammography alone using BCSC data, there was no difference in interval cancers (adjusted RR, 0.67 [95% CI, 0.33-1.37]), 73 although in both studies the confidence intervals were wide for this uncommon outcome. In the BCSC analysis, the rates of referral to biopsy and false-positive biopsy recommendations were twice as high and short interval follow-up was 3 times higher for the group screened with ultrasonography. 73
Response to Public Comment
A draft version of this recommendation statement was posted for public comment on the USPSTF website from May 9, 2023, to June 6, 2023. The USPSTF received many comments on the draft recommendation and appreciates all the thoughtful views and perspectives that were shared. Many comments agreed with the draft recommendation. Several comments suggested that there should be no upper age limit for breast cancer screening or that an upper age should be based on life expectancy. In response, the USPSTF notes that no trials of breast cancer screening enrolled women 75 years or older and an emulated trial showed no benefit to screening women aged 75 to 79 or 80 to 84. Some comments suggested that breast cancer screening should start prior to age 40 years, either for all women or for women who are at increased risk of breast cancer. Relatedly, some comments expressed that risk-based screening should be recommended. In response, the USPSTF would like to reiterate that no trials of breast cancer screening enrolled women younger than 39 years. Additionally, the USPSTF found no evidence on the benefits or harms of individualized breast cancer screening based on risk factors. Several randomized trials of risk-based screening are underway (eg, the WISDOM trial) that may provide valuable information regarding this question.
Several comments expressed that breast cancer screening should be recommended annually. In response, the USPSTF would like to reiterate that it did not identify any randomized trials directly comparing annual vs biennial screening. Two trials conducted in the 1980s to 1990s reported no difference in breast cancer mortality or breast cancer features such as tumor size, node positivity status, or histologic grade when comparing annual vs triennial screening. The USPSTF considers both the benefits and harms of different screening intervals and notes that the modeling studies commissioned to support this recommendation found that biennial screening results in greater life-years gained and mortality reduction per mammogram and has a more favorable balance of benefits to harms compared with annual screening.
Many comments requested that the USPSTF recommend supplemental screening with MRI or ultrasound for women with dense breasts. Some comments expressed that this would improve health outcomes, while other comments requested this recommendation so that supplemental screening would be covered by insurance. In response, the USPSTF wants to restate that it found insufficient evidence on the effects of supplemental screening on health outcomes. No studies of supplemental screening reported on health outcomes or on the incidence of and progression to advanced breast cancer over more than 1 round of screening. The USPSTF wants all women to be able to get the care they need and would like to clarify that the I statement is not a recommendation for or against supplemental screening in women with dense breasts. It fundamentally means that there is insufficient evidence to assess the balance of benefits and harms, or to recommend for or against supplemental screening, and that women should talk with their clinicians about what is best given their individual circumstances. The USPSTF is also calling for more research to help close this important evidence gap.
Some comments requested clarification of the patient population included in this recommendation, particularly as it relates to women with a family history of breast cancer or those with a genetic predisposition to increased breast cancer risk. In response, the USPSTF clarified that this recommendation applies to women with a family history of breast cancer but not those who have a genetic marker or syndrome or chest radiation exposure at a young age associated with a high risk of breast cancer. The USPSTF also clarified that it has an existing recommendation on risk assessment, genetic counseling, and genetic testing for BRCA-related cancer.
Some comments expressed that racial and ethnic disparities in breast cancer outcomes, especially in Black women, need to be comprehensively addressed. Related comments expressed that the higher breast cancer mortality that Black women experience is primarily related to their not receiving follow-up evaluation and treatment of the same timeliness and quality as White women, and that starting screening at age 40 years will not remedy this inequity. The USPSTF agrees that mitigating disparities in breast cancer mortality is crucial and highlights these disparities in the Disparities in Breast Cancer Outcomes and Implementation Considerations section of this recommendation statement. The USPSTF also agrees that improvements across the entire spectrum of breast cancer care are needed to reduce mortality for individuals experiencing disparities associated with lower breast cancer survival. For this recommendation, current evidence shows that screening for breast cancer starting at age 40 years will be of significant benefit to Black women. The USPSTF is also calling for more research to understand the underlying causes of why Black women are more likely to be diagnosed with breast cancers that have biomarker patterns that confer greater risk for poor health outcomes, to understand the causes of and ways to mitigate the higher mortality from breast cancer that Black women experience.
Some comments disagreed with the USPSTF B recommendation for screening women between the ages of 40 and 49 years, questioned the evidence to support this, or expressed that the current recommendation downplays the harms of screening. In response, the USPSTF has clarified that it uses modeling to complement trial and observational evidence when there is empirical (ie, trial) evidence of the benefit of a preventive service on health outcomes, as there is for breast cancer screening. Decision modeling can assist the USPSTF in assessing the magnitude of the benefits and harms of different screening strategies. The USPSTF carefully weighs both the benefits and harms of a preventive service as it makes its recommendations and currently concludes, as it has in the past, that the benefits of breast cancer screening outweigh the harms for women between the ages of 40 and 49 years. The most recent epidemiologic data reviewed by the USPSTF show greater incidence of breast cancer at younger ages, and decision modeling shows a greater magnitude of benefit for screening women between the ages of 40 and 49 years. The USPSTF considered both these lines of evidence as it issued its current B recommendation for biennial screening mammography for women aged 40 to 74 years.
Last, in response to comments, the USPSTF added the breast cancer screening recommendations from the American College of Radiology to the Recommendations of Others section.
See Table 3 for research needs and gaps related to screening for breast cancer.
The American Cancer Society recommends that women with an average risk of breast cancer should undergo regular screening mammography starting at age 45 years. It suggests that women aged 45 to 54 years should be screened annually, that women 55 years or older should transition to biennial screening or have the opportunity to continue screening annually, that women should have the opportunity to begin annual screening between the ages of 40 and 44 years, and that women should continue screening mammography as long as their overall health is good and they have a life expectancy of 10 years or longer. 74
The American College of Obstetricians and Gynecologists recommends that women at average risk of breast cancer should be offered screening mammography starting at age 40 years, using shared decision-making, and if they have not initiated screening in their 40s, they should begin screening mammography by no later than age 50 years. It recommends that women at average risk of breast cancer should have screening mammography every 1 or 2 years and should continue screening mammography until at least age 75 years. Beyond age 75 years, the decision to discontinue screening mammography should be based on shared decision-making informed by the woman’s health status and longevity. 75
The American College of Radiology and the Society of Breast Imaging recommend annual screening mammography beginning at age 40 years for women at average risk. They recommend that screening should continue past age 74 years, without an upper age limit, unless severe comorbidities limit life expectancy. 76 The American College of Radiology also recommends breast cancer risk assessment by age 25 years for all individuals. 77
The American Academy of Family Physicians supports the 2016 USPSTF recommendation on screening for breast cancer. 78
The authors of this recommendation statement include Task Force members serving at the time of publication and former members who made significant contributions to the recommendation. Any member with a level 3 conflict of interest (COI) recusal is not included as an author (see below for relevant COI disclosures for this topic).
The US Preventive Services Task Force authors of this recommendation statement include the following individuals: Wanda K. Nicholson, MD, MPH, MBA (George Washington University, Washington, DC); Michael Silverstein, MD, MPH (Brown University, Providence, Rhode Island); John B. Wong, MD (Tufts University School of Medicine, Boston, Massachusetts); Michael J. Barry, MD (Harvard Medical School, Boston, Massachusetts); David Chelmow, MD (Virginia Commonwealth University, Richmond); Tumaini Rucker Coker, MD, MBA (University of Washington, Seattle); Esa M. Davis, MD, MPH (University of Maryland School of Medicine, Baltimore); Carlos Roberto Jaén, MD, PhD, MS (University of Texas Health Science Center, San Antonio); M. (Tonette) Krousel-Wood, MD, MSPH (Tulane University, New Orleans, Louisiana); Sei Lee, MD, MAS (University of California, San Francisco); Li Li, MD, PhD, MPH (University of Virginia, Charlottesville); Carol M. Mangione, MD, MSPH (University of California, Los Angeles); Goutham Rao, MD (Case Western Reserve University, Cleveland, Ohio); John M. Ruiz, PhD (University of Arizona, Tucson); James Stevermer, MD, MSPH (University of Missouri, Columbia); Joel Tsevat, MD, MPH (University of Texas Health Science Center, San Antonio); Sandra Millon Underwood, PhD, RN (University of Wisconsin, Milwaukee); Sarah Wiehe, MD, MPH (Indiana University, Bloomington).
Conflict of Interest Disclosures: Authors followed the policy regarding conflicts of interest described at https://uspreventiveservicestaskforce.org/uspstf/about-uspstf/conflict-interest-disclosures . All members of the USPSTF receive travel reimbursement and an honorarium for participating in USPSTF meetings. Dr Wong reported delivering numerous unpaid talks on the 2009 USPSTF breast cancer screening recommendation; serving as a paid statistical reviewer for review of the USPSTF breast cancer screening models for the Annals of Internal Medicine in 2016 and of Cancer Intervention and Surveillance Modeling Network (CISNET) breast cancer and other cancer models submitted to the Annals of Internal Medicine ; serving as an unpaid National Cancer Institute chair of a group (2 others) that performed an external evaluation of the CISNET Program to assess its past productivity and adherence to its mission in 2018 (offered payment but never received); and serving as an unpaid member of the National Cancer Institute–convened Multi-cancer Early Detection (MCED) Diagnostic Pathways Working Group. Dr Chelmow reported serving as chair of the American College of Obstetricians and Gynecologists Practice Advisory Committee; in this role, he was involved in the development of practice guidelines related to breast cancer screening. Dr Barry reported receiving grants from Healthwise, a nonprofit, outside the submitted work. Dr Lee reported receiving grants from the National Institute on Aging (K24AG066998, R01AG079982) outside the submitted work. No other disclosures were reported.
No Task Force members had a level 3 COI recusal from this topic.
Funding/Support: The USPSTF is an independent, voluntary body. The US Congress mandates that the Agency for Healthcare Research and Quality (AHRQ) support the operations of the USPSTF.
Role of the Funder/Sponsor: AHRQ staff assisted in the following: development and review of the research plan, commission of the systematic evidence review from an Evidence-based Practice Center, coordination of expert review and public comment of the draft evidence report and draft recommendation statement, and the writing and preparation of the final recommendation statement and its submission for publication. AHRQ staff had no role in the approval of the final recommendation statement or the decision to submit for publication.
Disclaimer: Recommendations made by the USPSTF are independent of the US government. They should not be construed as an official position of AHRQ or the US Department of Health and Human Services.
Copyright Notice: USPSTF recommendations are based on a rigorous review of existing peer-reviewed evidence and are intended to help primary care clinicians and patients decide together whether a preventive service is right for a patient's needs. To encourage widespread discussion, consideration, adoption, and implementation of USPSTF recommendations, AHRQ permits members of the public to reproduce, redistribute, publicly display, and incorporate USPSTF work into other materials provided that it is reproduced without any changes to the work of portions thereof, except as permitted as fair use under the US Copyright Act.
AHRQ and the US Department of Health and Human Services cannot endorse, or appear to endorse, derivative or excerpted materials, and they cannot be held liable for the content or use of adapted products that are incorporated on other Web sites. Any adaptations of these electronic documents and resources must include a disclaimer to this effect. Advertising or implied endorsement for any commercial products or services is strictly prohibited.
This work may not be reproduced, reprinted, or redistributed for a fee, nor may the work be sold for profit or incorporated into a profit-making venture without the express written permission of AHRQ. This work is subject to the restrictions of Section 1140 of the Social Security Act, 42 U.S.C. §320b-10. When parts of a recommendation statement are used or quoted, the USPSTF Web page should be cited as the source.
1. Surveillance Epidemiology and End Results Program. Cancer Stat Facts: female breast cancer. National Cancer Institute. Accessed March 5, 2024. https://seer.cancer.gov/statfacts/html/breast.html 2. Surveillance Epidemiology and End Results Program. Breast: SEER 5-year age-adjusted incidence rates, 2016-2020, by race/ethnicity, female, all ages, all stages. National Cancer Institute. Accessed March 5, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=55&data_type=1&graph_type=10&compareBy=race&chk_race_6=6&chk_race_5=5&chk_race_4=4&chk_ race_9=9&chk_race_8=8&series=9&sex=3&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0#resultsRegion0 3. Surveillance Epidemiology and End Results Program. SEER*Stat Database: incidence—SEER research limited-field data with delay-adjustment, 22 registries, malignant only, November 2021 submission (2000-2019)—linked to county attributes—time dependent (1990-2019) income/rurality, 1969-2020 counties. National Cancer Institute. 2022. Accessed March 26, 2024. https://seer.cancer.gov/data-software/documentation/seerstat/nov2021/ 4. Henderson JT, Webber, EM, Weyrich M, Miller M, Melnikow J. Screening for Breast Cancer: A Comparative Effectiveness Review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 231. Agency for Healthcare Research and Quality; 2024. AHRQ publication 23-05303-EF-1. 5. Surveillance Epidemiology and End Results Program. Breast: SEER 5-year age-adjusted incidence rates, 2016-2020, by subtype, female, all races/ethnicities, all ages, all stages. National Cancer Institute. Accessed March 5, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=55&data_type=1&graph_type=10&compareBy=subtype&chk_subtype_55=55&chk_subtype_622=622&chk_subtype_623=623&chk_subtype_620=620&chk_subtype_621=621& series=9&sex=3&race=1&age_range=1&stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view 6. Surveillance Epidemiology and End Results Program. Breast: SEER 5-year age-adjusted mortality rates, 2016-2020, by race/ethnicity. National Cancer Institute. Accessed March 5, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=55&data_type=2&graph_type=10&compareBy=race&chk_race_6=6&chk_race_5=5&chk_race_4=4&chk_race_9=9&chk_race_8=8&series=9&sex=3&age_range=1&advopt_precision=1&advopt_show_ci=on &hdn_view=0&advopt_show_apc=on&advopt_display=2#resultsRegion 7. U.S. Preventive Services Task Force. Procedure Manual. Published May 2021. Accessed March 5, 2024. https://www.uspreventiveservicestaskforce.org/uspstf/about-uspstf/methods-and-processes/procedure-manual 8. US Preventive Services Task Force. Risk assessment, genetic counseling, and genetic testing for BRCA-related cancer: US Preventive Services Task Force recommendation statement . JAMA . 2019;322(7):652-665. Medline:31429903 doi:10.1001/jama.2019.10987 9. Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol . 2013;14(7):583-589. Medline:23623721 doi:10.1016/S1470-2045(13)70134-7 10. Skaane P, Bandos AI, Eben EB, et al. Two-view digital breast tomosynthesis screening with synthetically reconstructed projection images: comparison with digital breast tomosynthesis with full-field digital mammographic images. Radiology . 2014;271(3):655-663. Medline:24484063 doi:10.1148/radiol.13131391 11. Breast Cancer Surveillance Consortium. About the BCSC. Accessed March 5, 2024. https://www.bcsc-research.org/about 12. Trentham-Dietz A, Chapman CH, Jinani J, et al. Breast Cancer Screening With Mammography: An Updated Decision Analysis for the U.S. Preventive Services Task Force . Agency for Healthcare Research and Quality; 2024. AHRQ publication 23-05303-EF-2. 13. Trentham-Dietz A, Chapman CH, Jayasekera J, et al. Collaborative modeling to compare different breast cancer screening strategies: a decision analysis for the US Preventive Services Task Force. JAMA . Published April 30, 2024. doi:10.1001/jama.2023.24766 14. Miglioretti DL, Zhu W, Kerlikowske K, et al; Breast Cancer Surveillance Consortium. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status . JAMA Oncol . 2015;1(8):1069-1077. doi:10.1001/jama.2023.24766 15. Breast Cancer Treatment (PDQ®)—Health Professional Version. National Cancer Institute. Accessed April 10, 2024. https://www.cancer.gov/types/breast/hp/breast-treatment-pdq 16. Alvidrez J, Castille D, Laude-Sharp M, Rosario A, Tabor D. The National Institute on Minority Health and health disparities research framework. Am J Public Health . 2019;109(S1):S16-S20. Medline:30699025 doi:10.2105/AJPH.2018.304883 17. Williams DR, Priest N, Anderson NB. Understanding associations among race, socioeconomic status, and health: patterns and prospects. Health Psychol . 2016;35(4):407-411. Medline:27018733 doi:10.1037/hea0000242 18. Bailey ZD, Krieger N, Agénor M, Graves J, Linos N, Bassett MT. Structural racism and health inequities in the USA: evidence and interventions. Lancet . 2017;389(10077):1453-1463. Medline:28402827 doi:10.1016/S0140-6736(17)30569-X 19. Zavala VA, Bracci PM, Carethers JM, et al. Cancer health disparities in racial/ethnic minorities in the United States. Br J Cancer . 2021;124(2):315-332. Medline:32901135 doi:10.1038/s41416-020-01038-6 20. Bemanian A, Beyer KM. Measures matter: the local exposure/isolation (LEx/Is) metrics and relationships between local-level segregation and breast cancer survival. Cancer Epidemiol Biomarkers Prev . 2017;26(4):516-524. Medline:28325737 doi:10.1158/1055-9965.EPI-16-0926 21. Goel N, Westrick AC, Bailey ZD, et al. Structural racism and breast cancer-specific survival: impact of economic and racial residential segregation. Ann Surg . 2022;275(4):776-783. Medline:35081560 doi:10.1097/SLA.0000000000005375 22. Siegel SD, Brooks MM, Lynch SM, Sims-Mourtada J, Schug ZT, Curriero FC. Racial disparities in triple negative breast cancer: toward a causal architecture approach. Breast Cancer Res . 2022;24(1):37. Medline:35650633 doi:10.1186/s13058-022-01533-z 23. Niraula S, Biswanger N, Hu P, Lambert P, Decker K. Incidence, characteristics, and outcomes of interval breast cancers compared with screening-detected breast cancers. JAMA Netw Open . 2020;3(9):e2018179. Medline:32975573 doi:10.1001/jamanetworkopen.2020.18179 24. Jatoi I, Sung H, Jemal A. The emergence of the racial disparity in U.S. breast-cancer mortality. New Engl J Med . 2022;386(25):2349-2352. Medline:35713541 doi:10.1056/NEJMp2200244 25. Davis Lynn BC, Chernyavskiy P, Gierach GL, Rosenberg PS. Decreasing incidence of estrogen receptor-negative breast cancer in the United States: trends by race and region. J Natl Cancer Inst . 2022;114(2):263-270. Medline:34508608 doi:10.1093/jnci/djab186 26. Plevritis SK, Munoz D, Kurian AW, et al. Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000-2012. JAMA . 2018;319(2):154-164. Medline:29318276 doi:10.1001/jama.2017.19130 27. Fayanju OM, Ren Y, Stashko I, et al. Patient-reported causes of distress predict disparities in time to evaluation and time to treatment after breast cancer diagnosis. Cancer. 2021;127(5):757-768. Medline:33175437 doi:10.1002/cncr.33310 28. Selove R, Kilbourne B, Fadden MK, et al. Time from screening mammography to biopsy and from biopsy to breast cancer treatment among black and white, women Medicare beneficiaries not participating in a health maintenance organization. Womens Health Issues . 2016;26(6):642-647. Medline:27773529 doi:10.1016/j.whi.2016.09.003 29. Nguyen KH, Pasick RJ, Stewart SL, Kerlikowske K, Karliner LS. Disparities in abnormal mammogram follow-up time for Asian women compared with non-Hispanic white women and between Asian ethnic groups. Cancer . 2017;123(18):3468-3475. Medline:28603859 doi:10.1002/cncr.30756 30. Warner ET, Tamimi RM, Hughes ME, et al. Time to diagnosis and breast cancer stage by race/ethnicity. Breast Cancer Res Treat . 2012;136(3):813-821. Medline:23099438 doi:10.1007/s10549-012-2304-1 31. Kovar A, Bronsert M, Jaiswal K, et al. The waiting game: how long are breast cancer patients waiting for definitive diagnosis? Ann Surg Oncol . 2020;27(10):3641-3649. Medline:32314153 doi:10.1245/s10434-020-08484-9 32. Elmore JG, Nakano CY, Linden HM, Reisch LM, Ayanian JZ, Larson EB. Racial inequities in the timing of breast cancer detection, diagnosis, and initiation of treatment. Med Care . 2005;43(2):141-148. Medline:15655427 doi:10.1097/00005650-200502000-00007 33. Emerson MA, Golightly YM, Aiello AE, et al. Breast cancer treatment delays by socioeconomic and health care access latent classes in Black and White women. Cancer . 2020;126(22):4957-4966. Medline:32954493 doi:10.1002/cncr.33121 34. Lawson MB, Bissell MC, Miglioretti DL, et al. Multilevel factors associated with time to biopsy after abnormal screening mammography results by race and ethnicity. JAMA Oncol . 2022;8(8):1115-1126. Medline:35737381 doi:10.1001/jamaoncol.2022.1990 35. Hu X, Walker MS, Stepanski E, et al. Racial differences in patient-reported symptoms and adherence to adjuvant endocrine therapy among women with early-stage, hormone receptor-positive breast cancer. JAMA Netw Open . 2022;5(8):e2225485. Medline:35947386 doi:10.1001/jamanetworkopen.2022.25485 36. Hu X, Chehal PK, Kaplan C, et al. Characterization of clinical symptoms by race among women with early-stage, hormone receptor-positive breast cancer before starting chemotherapy. JAMA Netw Open . 2021;4(6):e2112076. Medline:34061200 doi:10.1001/jamanetworkopen.2021.12076 37. Clemons K, Blackford AL, Gupta A, et al. Geographic disparities in breast cancer mortality and place of death in the United States from 2003 to 2019. J Clin Oncol . 2022;40(16 Suppl):12034. doi:10.1200/JCO.2022.40.16_suppl.12034 38. Surveillance Epidemiology and End Results Program. Breast: SEER incidence rates by age at diagnosis, 2016-2020, by sex, delay-adjusted SEER incidence rate, all races/ethnicities. National Cancer Institute. Accessed March 5, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=55&data_type=1&graph_type=3&compareBy=sex&chk_sex_3=3&rate_type=2&race=1&advopt_precision=1& advopt_show_ci=on&hdn_view=0#resultsRegion0 39. Surveillance Epidemiology and End Results Program. Breast: U.S. Mortality Rates by Age at Death, 2016-2020, by Sex, All Races/Ethnicities. National Cancer Institute. Accessed March 5, 2024. https://seer.cancer.gov/statistics-network/explorer/application.html?site=55&data_type=2&graph_type=3&compareBy=sex&chk_sex_3=3&race=1 &advopt_precision=1&advopt_show_ci=on&hdn_view=0#resultsRegion0 40. García-Albéniz X, Hernán MA, Logan RW, Price M, Armstrong K, Hsu J. Continuation of annual screening mammography and breast cancer mortality in women older than 70 years. Ann Intern Med . 2020;172(6):381-389. Medline:32092767 doi:10.7326/M18-1199 41. Kerlikowske K, Zhu W, Tosteson AN, et al. Identifying women with dense breasts at high risk for interval cancer: a cohort study. Ann Intern Med . 2015;162(10):673-681. Medline:25984843 doi:10.7326/M14-1465 42. Price ER, Hargreaves J, Lipson JA, et al. The California breast density information group: a collaborative response to the issues of breast density, breast cancer risk, and breast density notification legislation. Radiology . 2013;269(3):887-892. Medline:24023072 doi:10.1148/radiol.13131217 43. Gierach GL, Ichikawa L, Kerlikowske K, et al. Relationship between mammographic density and breast cancer death in the Breast Cancer Surveillance Consortium. J Natl Cancer Inst . 2012;104(16):1218-1227. Medline:22911616 doi:10.1093/jnci/djs327 44. Nelson HD, Cantor A, Humphrey L, et al. Screening for Breast Cancer: A Systematic Review to Update the 2009 US Preventive Services Task Force Recommendation. Evidence Synthesis No. 124. Agency for Healthcare Research and Quality; 2016. AHRQ publication 14-05201-EF-1. 45. Centers for Disease Control and Prevention. Health, United States, 2018. Published 2018. Accessed March 5, 2024. https://www.cdc.gov/nchs/data/hus/hus18.pdf 46. State legislation map. Dense Breast-info. Accessed March 5, 2024. https://densebreast-info.org/legislative-information/state-legislation-map/ 47. Mammography Quality Standards Act, 21 C.F.R. § 900 (2023). 48. US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US Preventive Services Task Force recommendation statement. JAMA . 2019;322(9):857-867. Medline:31479144 doi:10.1001/jama.2019.11885 49. Siu AL; US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med . 2016;164(4):279-296. Medline:26757170 doi:10.7326/M15-2886 50. Henderson JT, Webber EM, Weyrich MS, Miller M, Melnikow J. Screening for breast cancer: evidence report and systematic review for the US Preventive Services Task Force. JAMA . Published April 30, 2024. doi:10.1001/jama.2023.25844 51. Owens DK, Whitlock EP, Henderson J, et al; US Preventive Services Task Force. Use of decision models in the development of evidence-based clinical preventive services recommendations: methods of the US Preventive Services Task Force. Ann Intern Med . 2016;165(7):501-508. Medline:27379742 doi:10.7326/M15-2531 52. Nyström L, Bjurstam N, Jonsson H, Zackrisson S, Frisell J. Reduced breast cancer mortality after 20+ years of follow-up in the Swedish randomized controlled mammography trials in Malmö, Stockholm, and Göteborg. J Med Screen . 2017;24(1):34-42. Medline:27306511 doi:10.1177/0969141316648987 53. Jones BA, Patterson EA, Calvocoressi L. Mammography screening in African American women: evaluating the research. Cancer . 2003;97(1 Suppl):258-272. Medline:12491490 doi:10.1002/cncr.11022 54. Parvinen I, Chiu S, Pylkkänen L, Klemi P, Immonen-Räihä P, Kauhava L, et al. Effects of annual vs triennial mammography interval on breast cancer incidence and mortality in ages 40-49 in Finland. Br J Cancer . 2011;105:1388-91. Medline:21934688 doi:10.1038/bjc.2011.372 55. Breast Screening Frequency Trial Group; United Kingdom Co-ordinating Committee on Cancer Research. The frequency of breast cancer screening: results from the UKCCCR randomised trial. Eur J Cancer . 2002;38(11):1458-1464. Medline:12110490 doi:10.1016/S0959-8049(01)00397-5 56. Armaroli P, Frigerio A, Correale L, et al. A randomised controlled trial of digital breast tomosynthesis versus digital mammography as primary screening tests: screening results over subsequent episodes of the Proteus Donna study. Int J Cancer . 2022;151(10):1778-1790. Medline:35689673 doi:10.1002/ijc.34161 57. Hofvind S, Moshina N, Holen ÅS, et al. Interval and subsequent round breast cancer in a randomized controlled trial comparing digital breast tomosynthesis and digital mammography screening. Radiology . 2021;300(1):66-76. Medline:33973840 doi:10.1148/radiol.2021203936 58. Pattacini P, Nitrosi A, Giorgi Rossi P, et al. A randomized trial comparing breast cancer incidence and interval cancers after tomosynthesis plus mammography versus mammography alone. Radiology . 2022;303(2):256-266. Medline:35103537 doi:10.1148/radiol.211132 59. Hovda T, Holen ÅS, Lång K, et al. Interval and consecutive round breast cancer after digital breast tomosynthesis and synthetic 2d mammography versus standard 2d digital mammography in BreastScreen Norway. Radiology . 2020;294(2):256-264. Medline:31821118 doi:10.1148/radiol.2019191337 60. Ho TH, Bissell MC, Kerlikowske K, et al. Cumulative probability of false-positive results after 10 years of screening with digital breast tomosynthesis vs digital mammography. JAMA Netw Open . 2022;5(3):e222440 Medline:35333365 doi:10.1001/jamanetworkopen.2022.2440 61. McGuinness JE, Ueng W, Trivedi MS, et al. Factors associated with false positive results on screening mammography in a population of predominantly Hispanic women. Cancer Epidemiol Biomarkers Prev . 2018;27(4):446-453. Medline:29382701 doi:10.1158/1055-9965.EPI-17-0009 62. Conant EF, Beaber EF, Sprague BL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography compared to digital mammography alone: a cohort study within the PROSPR consortium. Breast Cancer Res Treat . 2016;156(1):109-116. Medline:26931450 doi:10.1007/s10549-016-3695-1 63. Kerlikowske K, Su YR, Sprague BL, et al. Association of screening with digital breast tomosynthesis vs digital mammography with risk of interval invasive and advanced breast cancer. JAMA . 2022;327(22):2220-2230. Medline:35699706 doi:10.1001/jama.2022.7672 64. Richman IB, Long JB, Hoag JR, et al. Comparative effectiveness of digital breast tomosynthesis for breast cancer screening among women 40-64 years old. J Natl Cancer Inst . 2021;113(11):1515-1522. Medline:33822120 doi:10.1093/jnci/djab063 65. Johnson K, Lang K, Ikeda DM, et al. Interval breast cancer rates and tumor characteristics in the prospective population-based Malmö breast tomosynthesis screening trial. Radiology . 2021;299(3):559-567. Medline:33825509 doi:10.1148/radiol.2021204106 66. Zackrisson S, Lång K, Rosso A, et al. One-view breast tomosynthesis versus two-view mammography in the Malmö Breast Tomosynthesis Screening Trial (MBTST): a prospective, population-based, diagnostic accuracy study. Lancet Oncol . 2018;19(11):1493-1503. Medline:30322817 doi:10.1016/S1470-2045(18)30521-7 67. Heindel W, Weigel S, Gerß J, et al. Digital breast tomosynthesis plus synthesized mammography versus digital screening mammography for the detection of invasive breast cancer (TOSYMA): a multicentre, open-label, randomised, controlled, superiority trial. Lancet Oncol . 2022;23(5):601-611. Medline:35427470 doi:10.1016/S1470-2045(22)00194-2 68. Aase HS, Holen ÅS, Pedersen K, et al. A randomized controlled trial of digital breast tomosynthesis versus digital mammography in population-based screening in Bergen: interim analysis of performance indicators from the To-Be trial. Eur Radiol. 2019;29(3):1175-1186. Medline:30159620 doi:10.1007/s00330-018-5690-x 69. Bakker MF, de Lange SV, Pijnappel RM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med . 2019;381(22):2091-2102. Medline:31774954 doi:10.1056/NEJMoa1903986 70. Veenhuizen SG, de Lange SV, Bakker MF, et al. Supplemental breast MRI for women with extremely dense breasts: results of the second screening round of the DENSE trial. Radiology . 2021;299(2):278-286. Medline:33724062 doi:10.1148/radiol.2021203633 71. Ganguli I, Keating NL, Thakore N, Lii J, Raza S, Pace LE. downstream mammary and extramammary cascade services and spending following screening breast magnetic resonance imaging vs mammography among commercially insured women. JAMA Netw Open . 2022;5(4):e227234. Medline:35416989 doi:10.1001/jamanetworkopen.2022.7234 72. Ohuchi N, Suzuki A, Sobue T, et al; J-START Investigator Groups. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet . 2016;387(10016):341-348. Medline:26547101 doi:10.1016/S0140-6736(15)00774-6 73. Lee JM, Arao RF, Sprague BL, et al. Performance of screening ultrasonography as an adjunct to screening mammography in women across the spectrum of breast cancer risk. JAMA Intern Med . 2019;179(5):658-667. Medline:26547101 doi:10.1016/S0140-6736(15)00774-6 74. Oeffinger KC, Fontham ET, Etzioni R, et al; American Cancer Society. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA . 2015;314(15):1599-1614. Medline:26501536 doi:10.1001/jama.2015.12783 75. Committee on Practice Bulletins—Gynecology. Practice Bulletin Number 179: breast cancer risk assessment and screening in average-risk women. Obstet Gynecol . 2017;130(1):e1-e16. Medline:28644335 doi:10.1097/AOG.0000000000002158 76. Monticciolo DL, Malak SF, Friedewald SM, et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol . 2021;18(9):1280-1288. Medline:34154984 doi:10.1016/j.jacr.2021.04.021 77. Monticciolo DL, Newell MS, Moy L, Lee CS, Destounis SV. Breast cancer screening for women at higher-than-average risk: updated recommendations from the ACR. J Am Coll Radiol . 2023;20(9):902-914. Medline:37150275 doi:10.1016/j.jacr.2023.04.002 78. American Academy of Family Physicians. Clinical Preventive Service Recommendation: Breast Cancer. Accessed March 5, 2024. https://www.aafp.org/family-physician/patient-care/clinical-recommendations/all-clinical-recommendations/breast-cancer.html
Abbreviations: MRI, magnetic resonance imaging; USPSTF, US Preventive Services Task Force.
Abbreviations: DBT, digital breast tomosynthesis; DCIS, ductal carcinoma in situ; MRI, magnetic resonance imaging; USPSTF, US Preventive Services Task Force.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Epidemiology
- Open access
- Published: 26 October 2021
The current status of risk-stratified breast screening
- Ash Kieran Clift ORCID: orcid.org/0000-0002-0061-979X 1 , 2 ,
- David Dodwell 3 ,
- Simon Lord ORCID: orcid.org/0000-0001-7946-5609 4 ,
- Stavros Petrou 2 ,
- Sir Michael Brady 4 ,
- Gary S. Collins ORCID: orcid.org/0000-0002-2772-2316 5 , 6 &
- Julia Hippisley-Cox ORCID: orcid.org/0000-0002-2479-7283 2
British Journal of Cancer volume 126 , pages 533–550 ( 2022 ) Cite this article
10k Accesses
48 Citations
29 Altmetric
Metrics details
- Breast cancer
- Cancer epidemiology
Apart from high-risk scenarios such as the presence of highly penetrant genetic mutations, breast screening typically comprises mammography or tomosynthesis strategies defined by age. However, age-based screening ignores the range of breast cancer risks that individual women may possess and is antithetical to the ambitions of personalised early detection. Whilst screening mammography reduces breast cancer mortality, this is at the risk of potentially significant harms including overdiagnosis with overtreatment, and psychological morbidity associated with false positives. In risk-stratified screening, individualised risk assessment may inform screening intensity/interval, starting age, imaging modality used, or even decisions not to screen. However, clear evidence for its benefits and harms needs to be established. In this scoping review, the authors summarise the established and emerging evidence regarding several critical dependencies for successful risk-stratified breast screening: risk prediction model performance, epidemiological studies, retrospective clinical evaluations, health economic evaluations and qualitative research on feasibility and acceptability. Family history, breast density or reproductive factors are not on their own suitable for precisely estimating risk and risk prediction models increasingly incorporate combinations of demographic, clinical, genetic and imaging-related parameters. Clinical evaluations of risk-stratified screening are currently limited. Epidemiological evidence is sparse, and randomised trials only began in recent years.
Similar content being viewed by others

Genome-wide association studies

Segment anything in medical images

Causal machine learning for predicting treatment outcomes
Introduction.
Breast screening is widely implemented in many healthcare systems to reduce breast cancer mortality through the expedited diagnosis of smaller, asymptomatic breast cancers. For the majority of women, this uses mammography starting in middle age, although different regions differ in their screening recommendations and practices (Table 1 ). In rarer, very high-risk situations such as a known, high-penetrance genetic predisposition, earlier screening with magnetic resonance or ultrasound imaging is advocated [ 1 , 2 ].
Whilst meta-analysis of randomised clinical trial data clearly demonstrates a reduction in the relative risk of breast cancer mortality due to screening, reduced breast cancer deaths may come at the expense of overdiagnosis (the identification and unnecessary treatment of clinically insignificant tumours), as well as the consequences of false-positive or false-negative results [ 3 , 4 , 5 , 6 , 7 , 8 ]. There is wide variability in the results seen in observational studies in relation to overdiagnosis estimations, depending on analytical or modelling approaches [ 9 , 10 , 11 , 12 ]. The summation of evidence assessed by the UK Independent Panel [ 3 ] was that breast screening does reduce mortality. It concluded that for every 10,000 women in the United Kingdom aged 50 years invited to screening for the next 20 years, 43 breast cancer deaths would be avoided and 681 tumours (invasive or ductal carcinoma in situ [DCIS]) would be diagnosed, but 129 women would be overdiagnosed, i.e. three overdiagnosed cases per breast cancer death averted, although this calculated benefit–harm balance has been contested by some [ 13 , 14 ].
Most countries use an age-based population-level breast screening strategy that reduces breast cancer mortality but does not account for the wide variation in individual women’s cancer risks [ 15 , 16 , 17 , 18 ]. Identification of women at the highest risk of breast cancer may enable targeted intensification of early detection or preventive measures, and reduce the public health burden of this common malignancy. Motivated by such factors, ‘risk-stratified’ breast screening has emerged as a concept in which decisions to offer screening or the determination of screening frequency and modality (e.g. from mammography/tomosynthesis to magnetic resonance imaging [MRI]) are guided by accurate estimation of an individual woman’s risk of breast cancer [ 15 , 19 , 20 , 21 ]. Logically, risk-stratified screening would target intervention at those that stand to gain the most and reduce or stop screening in those that stand to gain little benefit, potentially also informed by the cost-effectiveness evidence.
However, the efficacy and feasibility of personalising screening strategies is uncertain and would require the meeting of several critical dependencies for implementation [ 21 ]. These include the need for rigorously developed and validated risk prediction models capable of stratifying women accurately, robust health economic evaluation, and clarity on the balance sheet of benefits and harms that would become the ‘new norm’ in clinical practice after prospective studies. Although some recent reviews have sought to summarise this field [ 21 , 22 , 23 ], they differ in the extent to which they cover each of these dependencies. In this narrative scoping review, the authors seek to provide a concise overview of the key topics within the risk-stratified screening literature and anticipate the potential effects of emerging evidence on clinical practice. First, the evidence for risk prediction modelling that may guide personalised screening is reviewed. Thereafter, the evidence from observational analyses of epidemiological or registry data is appraised prior to discussion of how ongoing prospective cohorts and trials have been designed in this area. Furthermore, health economic evidence and output from qualitative studies is synthesised. Throughout, there is an emphasis on evidence quality, its limitations and discussion of how unmet needs may be satisfied.
A scoping literature review was undertaken using Medline (PubMed) with the following search strategy: (“breast screening” OR “mammography”) AND (“risk#adapted” OR “risk#stratified” OR “personalised” OR “personalized” OR “tailored” OR “risk#based”). Papers published in any language prior to 1 November 2020 were considered for inclusion in this review. We reviewed the reference lists of systematic reviews to identify key publications if not identified by the search strategy. We also searched clinicaltrials.gov on 1 November 2020 to identify ongoing interventional studies in this area (for search terms: “breast cancer”, “screening” and “risk”).
Reports retrieved were screened for inclusion based on title and abstract and, if relevant, were classified into five groups: papers reporting risk prediction models, epidemiological analyses of risk-stratified screening or retrospective evaluations, prospective studies and trials of risk-stratified screening, health economic evaluations and qualitative research on feasibility or acceptability. Findings were synthesised narratively, informed by the narrative synthesis guidelines developed by the Cochrane Collaboration [ 24 ].
Risk prediction models to guide personalised screening
Several risk prediction models for breast cancer incidence have been reported, which tend to incorporate ‘classical’ risk factors identified from epidemiological evidence, e.g. clinical, demographic or pharmacological exposures, but may also assimilate factors such as family history, genetic risk markers or polygenic risk scores and imaging-related parameters. Well-recognised ‘risk factors’ include breast density, first-degree family history of breast cancer, increased body mass index (BMI), nulliparity or young age at first birth, and such factors may be attributable for over 52% of risk [ 25 ]. The role of obesity relative to menopause, such as the apparent ‘protective effect’ of obesity on oestrogen receptor-positive cancers prior to menopause [ 26 , 27 ], may be relevant but is not always incorporated into risk models [ 28 ].
Some models have also sought to incorporate markers of genetic risk, such as the inclusion of BRCA genotype in the Tyrer–Cuzick (also known as the ‘IBIS’) model [ 29 ], as well as the incorporation of a polygenic risk estimation incorporated into a later update thereof [ 30 ]. Some comprise predominantly genetic information, such as BOADICEA [ 31 , 32 , 33 ] and BRCAPRO [ 32 , 34 ]. Genetic predisposition, through either highly penetrant mutations such as BRCA1/2 [ 35 , 36 ] or subtler single-nucleotide polymorphisms (SNPs) may indeed affect breast cancer risk; however, only 25–50% of the familial risk can be explained by known genetic variants [ 35 , 37 , 38 , 39 , 40 ], and only 16% of the risk of nonfamilial breast cancer is accounted for by SNPs [ 36 ]. Furthermore, breast density and textural features [ 41 ] may be relevant to breast cancer risk and have been explored as covariates in risk prediction models rooted in mammographic image analysis or as additions to covariate panels in updated versions of statistical models. Generally, assessment of breast density could be determined by visual assessment scales [ 42 ], or automated/algorithmic approaches [ 43 , 44 , 45 ], but the most common appears to be the four-category ‘Breast Imaging Reporting and Data System’ classification [ 46 ] (BI-RADS) [ 30 , 47 , 48 ]. Modelling approaches utilised include mathematical modelling [ 29 ], statistical regression [ 49 , 50 ] or ‘machine learning’ techniques [ 51 ].
Whilst the implementation of risk-stratified screening to entire populations depends on the prospective outcome and economic evaluations, the requirement for accurate and robust multivariable risk prediction models is the sine qua non of any such approach [ 52 ]. All risk prediction models intended to be used to inform clinical decision-making should be transparently reported, and robustly evaluated in terms of various performance metrics [ 53 ]. Strong internal validation is recommended, and appropriate external validation using data sources that are independent of those used to generate the model may also be useful [ 54 , 55 ]. Important considerations include the discrimination of models, i.e. how well they distinguish between women who do develop breast cancer versus those who do not [ 56 ]; calibration, i.e. the degree of agreement between the predicted risks and the observed risks [ 57 ]; and assessment of ‘net benefit’ using decision-curve analysis [ 58 , 59 ]. In terms of discrimination, the ‘area under the curve’ (AUC) or identical ‘ c -statistic’ may be used for binary outcomes, or the ‘ c -index’ for survival data [ 60 ] both range between 0 and 1, with values of 1 corresponding to perfect prediction and 0.5 reflecting discrimination no better than a coin toss. Other metrics to consider include the proportion of variance explained by the model, such as the R 2 [ 2 , 61 ]. It is increasingly clear that average performance metrics or ‘overall’ assessments of model performance in populations are insufficient to truly assess clinical utility on deployment, as there may be differences in model performance between regions, ethnic groups or even age groups [ 62 , 63 ].
Table 2 describes the development and validation results regarding key published risk prediction models. Our search strategy sought to identify original reports and secondary validation studies without restricting the latter to those performed by model developers. Interestingly, a recent systematic review by Louro et al. [ 64 ], which appraised the evidence for the Breast Cancer Risk Assessment Tool (BCRAT), Breast Cancer Surveillance Consortium (BCSC), Rosner and Colditz, IBIS and other models using the ISPOR-AMCP-NPC [ 65 ] questionnaire rather than PROBAST [ 66 ] found that it was challenging to recommend any model for the purposes of risk-stratified screening. Importantly, some risk prediction models were missed by their search strategy [ 50 , 67 ], and models focussing on genetic determinants of risk were intentionally excluded [ 64 ]. The emergent pattern is that incorporation of multiple data forms, such as adding breast density or other mammographic features, or genetic information yields incremental gains in model performance [ 68 ], although these tend to be relatively small [ 30 , 69 ]. Specifically, regarding incorporating breast density on discrimination, the increase in the AUC of published models ranges from 0.03 to 0.14 [ 69 ]. The effects on calibration are less clear and the effects on net benefit are not reported.
It is important to note that simply comparing AUC/ c -indices of different model development studies does not constitute a meaningful comparison as a breast cancer risk is strongly influenced by age, and the AUC/ c -index may be influenced by the heterogeneity of study population, the prediction horizons used and the source of the population used may differ across studies. For example, the AUC/ c -index of a model developed in a cohort with a very broad age range is not directly transferable to a separate cohort of women with a narrower age range, such as women eligible for screening currently.
The O/E ratio is widely used to assess calibration, and simply compares the overall number of observed cases versus the number predicted by a model for a given population, and as a standalone metric is insufficient, as over-prediction in sub-groups can be compensated by under-prediction in others and vice versa [ 57 ]. A more comprehensive analysis of alignment between predicted versus observed risks for individual study participants could include the use of a calibration plot displaying (mis)alignment across levels of risk [ 70 ]. Some papers report as one aspect of their analyses the hazard ratio of pre-selected highest risk groups (such as top tenth) to middle-risk groups (such as middle 80%) [ 47 ]. This alone may provide an incomplete assessment of model performance as it is comparing small groups at the extremes of a risk score distribution to the bulk of the study population, which would naturally be expected to diverge in their observed risk. These must therefore be interpreted in the context of the other sources of information regarding model performance when provided. A key external validation study of four key models in a cohort of 15,732 women from Australia, Canada and the US (519 cases of breast cancer) demonstrated c -statistics of 0.70 for BOADICEA (95% confidence interval [CI]: 0.68–0.72), 0.71 for IBIS (95% CI: 0.69–0.73), 0.68 for BRCAPRO (95% CI: 0.65–0.70) and 0.60 for BRCAT (0.58–0.62) [ 71 ]. Assessment of calibration was limited to the O/E ratio: BOADICEA 1.05 (95% CI: 0.97–1.14), IBIS 1.03 (95% CI: 0.96–1.12), BRCAPRO 0.68 (95% CI: 0.65–0.70), and BCRAT 0.79 (95% CI: 0.73–0.85).
Recently, there has been increasing interest in ‘machine learning’ prediction modelling for healthcare. Whilst arguably perceived to be more flexible (e.g. better at capturing non-linear, complex interactions), less reliant on assumptions than traditional regression and capable of handling some forms of data that regression models cannot, machine learning has not been shown to be inherently better than traditional statistical modelling approaches [ 72 ]. Datasets used for ML modelling should capture clinical reality, i.e. reflect the target population, and the architecture of any algorithm should be reported, given their structural flexibility. Clarity of reporting model development can be problematic [ 73 ] and validation/performance assessment approaches may not always be appropriate or transparent, especially when comparing different approaches. One recent study compared the performance of the BOADICEA model with a Markov chain Monte Carlo generalised linear mixed model, an adaptive boosting model and a random forest model developed using data from a single oncogenetic institution in Switzerland that focusses on counselling and testing for hereditary cancer syndromes [ 51 ]. Whilst the machine learning models were declared to outperform BOADICEA, no robust evaluation of model calibration was performed, and the effective comparison was an external validation of BOADICEA versus an internal validation of the new model using the data they were derived from. This used cross-validation with a low number of repeats ( n = 20), which presumably was used for hyperparameter tuning as well as performance evaluation (not elaborated in paper), an option that is optimistically biased [ 74 , 75 ]. Further work in this area of comparing different model-building strategies for predicting risk should focus on more robust, meaningful comparisons.
Overall, a range of clinical prediction models has been developed that could be used to guide risk-stratified screening, some of which are undergoing evaluation in trials of personalised screening. The ability of models to guide risk-stratified screening by predicting incident breast cancer risk in asymptomatic women is uncertain [ 64 ], even if integrating clinical, genetic and imaging-derived variables [ 30 , 47 , 49 , 68 ]. There is no single accepted benchmark for a given performance metric to render a model suitable for guiding personalised screening, and decisions regarding optimal models should not be made on a single metric. Instead, models need to be robustly assessed in terms of discrimination, calibration and potential clinical utility in the target populations. Whilst a model with an AUC of 0.5 in its target population cannot be informative, a high AUC in a model development/evaluation study does not guarantee utility in guiding risk-based screening. Poorly calibrated models may cause harm, and those with unstable performance across sub-groups may raise concerns regarding ‘algorithmic fairness/bias’. Some of these models are not fully developed using individual-level data, rather, are pre-determined systems of weights that are then applied to a test dataset to assess performance [ 30 , 47 ]. Crucially, however, weak or non-existent calibration assessment, non-examination of performance heterogeneity, or the lack of consideration of geographical and temporal transportability [ 55 , 76 ] are notable limitations. The QCancer (Breast) [ 50 ], IBIS [ 47 ] and iCARE [ 77 ] models are some examples wherein exploration of performance heterogeneity is performed according to age groups or other clinically relevant sub-populations (see Table 2 ). It has also been suggested that in order to minimise harms from overdiagnosis of indolent tumours, modelling the risk of developing lethal breast cancers could be more appropriate than modelling the diagnosis of any breast cancer in order to risk-stratify screening [ 11 ]. This is another avenue for further exploration and analysis.
Epidemiological analyses and retrospective evaluations of risk-stratified screening
The real-world effects of implementing risk-stratified screening strategies [ 78 ] warrant evaluation in prospective studies and trials. Whilst trials have recently been initiated (see later section), there have also been several explorations of the possible benefits and harms of using epidemiological approaches in a jurisdiction where multiple forms of mammography screening are available, namely in Taiwan. This complements studies modelling breast cancer risk in large cohort studies relative to age, or retrospectively simulating the effect of implementing different screening practices in screening cohorts. Table 3 summarises the evidence from such epidemiological papers or retrospective clinical evaluations simulating the possible effects of risk decision rules using patient data.
A large Taiwanese study ( n > 1.4 million) exploited the natural experiment of the concurrent availability of three screening approaches in the country’s population, namely annual clinical breast examination as the baseline (women aged 35 years and over), risk-stratified biennial mammography screening or universal mammography (both for women aged 50–69 years) [ 79 ]. The existence of three available approaches was predicated by a low breast cancer incidence rate in 2002–2004 and concerns about healthcare system capacity for whole population screening at the time, although the rates have increased since [ 80 ]. Risk stratification used a ‘risk score’ derived from reproductive/menstrual history and family history data obtained during attendances for clinical breast examination between 1999 and 2001, with the median of the risk scores used as the cut-off for eligibility for biennial mammography. Using propensity score methodology to try to adjust for disparities in baseline risk factors across the three groups (age at menarche, parity, breastfeeding and BMI), Cox models were used, where screening modality was modelled as a time-dependent covariate. Compared to clinical examination, universal biennial mammography had a higher breast cancer detection rate, was associated with a downwards stage migration of detected cancers, a 13% overdiagnosis rate (95% CI: 8–18%), a 30% reduction in stage II + breast cancers (hazard ratio [HR] 0.70, 95% CI: 0.66–0.74), and a 41% reduction in breast cancer mortality (95% CI: 27–52%) when adjusting for propensity score and year of birth [ 79 ]. Compared to clinical examination, the overdiagnosis with risk-based screening was negligible (HR for diagnosis 0.97, 95% CI: 0.92–1.03), there was an 8% reduction in stage II + breast cancers (HR 0.92, 95% CI: 0.86–0.99) and a ‘non-significant’ reduction in breast cancer mortality of 14% (HR 0.86, 95% CI: 0.73–1.03) [ 79 ]. However, the risk-stratification mechanism in this study was unclear—data were not provided on the modelling methodology used, the risk score covariates, the risk score distribution in the population seeking to opt into risk-based screening (or across the three arms) or performance evaluation to assess if this approach was suitable for clinical use. Further detail is needed to make meaningful inferences on the performance of risk-based screening versus ‘standard’ screening in this analysis. In addition, given the relatively low, albeit increasing, breast cancer incidence rate in this population, risk stratification beyond age and sex may have different proportional benefits in Taiwan in comparison to other nations.
Rather than analysing the effects of altering screening intensity or avoiding screening in low-risk women, a large Swedish cohort with linkage to several national databases ( n > 5,000,000 women) was used to assess whether earlier screening starting ages could be appropriate for some women. By using 10-year cumulative risk estimations, the risk level of the ‘average’ 50-year-old woman that would be offered screening was calculated as a benchmark. The ages at which other women would attain the same 10-year risk were compared, based on patterns of family history [ 81 ] or personal reproductive history (parity and age at first birth) [ 82 ]. Both studies found that either approach could identify women who, despite not being eligible to start age-based screening, had the same 10-year risk estimate as 50-year-old women who would be invited to screen, or indeed may only attain that same threshold of risk after age 50 years. For example, women who had their first baby aged under 25 years met the benchmark aged 51 years, whereas women who had four births by age 25 years met this at 59 years of age [ 82 ]. Furthermore, women with one first-degree relative diagnosed with breast cancer before the age of 40 years met the average risk of women starting age-based screening at age 36 years [ 81 ]. Therefore, despite debate around the benefits of universally expanding screening to younger age groups such as the lack of long-term effect seen in Age UK [ 83 ], selected women with selected risk factors may be suitable for earlier or delayed commencement of early detection strategies. The optimal way to assess risk would require elucidation as reliance on two albeit important risk factors may inadequately capture risk.
In the radiological literature, some commentators have voiced criticism of the potential harms offered by risk-stratified screening [ 84 , 85 , 86 ], typically fuelled by retrospective studies applying risk factor-based decision rules to cohorts of women that partook in service screening [ 86 , 87 , 88 , 89 ]. For example, Lee et al. examined recall rates, cancer detection rates and positive predictive values for biopsy recommendation and the fact of biopsy across age groups, when accounting for breast cancer family history, personal breast cancer history and having dense breasts in a cohort of >2.6 million women [ 88 ]. The recall and cancer detection rates in 30–39-year-old women were the same with these risk factors undergoing incidence screening as the 40–49-year-old ‘average-risk’ women undergoing screening; thus, they concluded that such a higher-risk women may benefit from earlier screening starting age. Other institutional studies have expressed concern that risk-stratified approaches have the potential to miss 75.6–88% of cancers if screening was purely based on family history, 56–86% of cancers if density was the sole determinant or 43.5–76% if access to screening was dictated by positive family history and breast density [ 86 , 89 ]. Such approaches are not powered to assess the effects of varying screening approaches on stage at detection nor breast cancer mortality, but most crucially, they rely on relatively simplistic determinations of relative risk. Prospective clinical trials are not evaluating such approaches, rather, more nuanced methods for estimating risk.
Overall, currently available epidemiological evaluations or retrospective clinical estimations of breast cancer screening guided by individualised risk are insufficient to inform the utility of risk-stratified screening.
Prospective cohort studies
Three notable cohort studies are currently exploring multiple aspects pertaining to the feasibility and acceptability of personalised risk assessment in the general population: specifically, the Personalised RISk-based MAmma screening study (PRISMA), the Karolinska Mammography Project for Risk Prediction of Breast Cancer (KARMA) and the Predicting the Risk of Cancer at Screening study (PROCAS).
PRISMA is a Dutch collaboration between institutions including Radboud UMC Nijmegen and the North, East, West and South Screening Programmes. In 2014, PRISMA started recruiting asymptomatic women aged 50–75 years in the general population eligible for the national screening programme for data collection via questionnaires, blood and saliva samples and mammograms for assessing breast density. It has a target of 90,000 women with regards to risk factor questionnaire data collection and imaging, and 27,000 blood samples. It aims to not only develop risk prediction models as a fulcrum for investigating risk-based screening strategies but undertake a robust assessment of the acceptability of risk-based screening from ethical, psychological, legal and logistical perspectives. Outputs from PRISMA thus far include multi-cohort qualitative research incorporating individuals from KARMA and PROCAS, which identified a preference for risk-tailored assessment results communication (e.g. letters for below average and average risk, face-to-face appointments for higher risk), the need for standardised risk assessments within national policies and detailed information needs for women in different European countries [ 90 , 91 ].
The KARMA prospective screening cohort is developing an extensive resource of banked biological, mammogram and lifestyle/clinical factor information from over 70,000 women [ 92 ]. Its aims include identification of novel circulating risk markers, genetic risk factors and imaging protocols, assessment of high-throughput breast density measurement, trials of pharmacological prevention therapies such as lower-dose anti-oestrogen therapy and risk communication as well as the development of new risk prediction models with an evaluation of how these could be implemented within screening routines. Notable outputs include the CAD2Y model, which integrates mammographic features such as computer-detected microcalcifications with ‘clinical’ factors for short-term risk prediction [ 93 ], risk estimation integrating mammographic density and polygenic risk [ 48 , 94 ], and contributions to the identification and understanding of genomic breast cancer risk by multi-centre consortia [ 95 , 96 , 97 , 98 ].
The PROCAS 2 study began in 2015 following PROCAS 1, which recruited over 50,000 women eligible for screening mammography at the Great Manchester NHS Breast Screening Programme. Lifestyle, reproductive history and other clinical informations were collected via questionnaires, with imaging assessment of mammographic density and DNA obtained for polygenic risk analysis [ 99 ]. Numerous studies have been undertaken within the remit of PROCAS, such as the evaluation of Tyrer–Cuzick and Gail risk prediction models in a screening population, the predictive impact of the inclusion of mammographic density [ 68 ] and/or polygenic risk score components into these [ 30 , 100 ], extensive assessment of risk feedback and perception [ 101 , 102 , 103 ] and probing associations between ethnicity and mammographic density [ 99 ]. PROCAS outputs have been central to supporting the feasibility of population-based breast cancer risk assessment [ 101 , 104 ], including identifying no major psychological harms of providing 10-year risk estimates from different forms of risk algorithms [ 103 ].
Prospective evaluations and trials of risk-stratified screening
Prospective clinical studies and ideally randomised trials should evaluate risk-based screening practices in terms of outcomes, balance of harms and benefits, cost-effectiveness and acceptability. Several key studies are underway [ 105 , 106 , 107 , 108 ], including NCT04359420 [ 108 ]. This is a non-randomised, counterbalanced study across seven screening sites in the United Kingdom, in which women on an invitation to the NHS Breast Screening Programme will either be offered the standard programme or the additional invitation to use BC-Predict, an automated system for offering a breast cancer risk assessment (to include questionnaires, breast density measurement and polygenic risk) on an invitation to screen [ 108 ]. Its aims include assessing risk assessment uptake after offer, uptake of risk consultation, chemoprevention or additional mammography, as well as risks of potential cancer worry, anxiety or health service costs [ 108 ]. Many others do not strictly seek to evaluate the outcomes of screening intensity/eligibility decisions based on individualised risk estimation. Indeed, some are also exploring how best to communicate personal risk (e.g. PROSPR/PCIPS 3, NCT01879189), promote breast screening uptake based on risk factor-specific educational materials (e.g. NCT00416975) or identify optimal imaging modalities for women at specific levels of predicted risk (e.g. NCT00003736).
The Women Informed to Screen Depending on Measures of risk (WISDOM) trial is a preference-tolerant randomised trial of a risk-based screening algorithm versus standard screening practice in the United States that started in 2016 [ 105 ]. Absolute breast cancer risk estimates are generated using the BCSC model [ 49 ], modified by a polygenic risk score incorporating 96 SNPs using Bayesian principles [ 105 ] and testing for nine high- or moderate-risk genes ( BRCA1 , BRCA2 , TP53 , STK11 , PTEN , CHD1 , ATM , PALB2 and CHEK2 ). Predicted risks at 5 years and age dictate the screening strategy. For those aged 40–49 years: women with a 5-year risk of <1.3% are not being offered screening, those with a 5-year risk of 1.3% or greater are undergoing biennial mammography, whilst women with extremely dense breasts or who are carriers of ATM/PALB2/CHEK2 mutations without a positive family history are undergoing annual mammography. For women aged 50–74 years, all are undergoing biennial mammography unless they are carriers of ATM/PALB2/CHEK2 mutations without a positive family history in which case they receive annual mammography. Regardless of age group, annual mammography with adjunct MRI is being deployed in carriers of BRCA1/BRCA2/TP53/PTEN/STK11/CDH1 mutations regardless of family history, carriers of ATM/PALB2/CHEK2 mutations with a positive family history, those who had chest irradiation between the ages of 10–30 years or have a 5-year breast cancer risk of at least 6%. With target recruitment of 100,000 women, it has been projected that 75% of women aged 40–49 years will be allocated to ‘no screening’, whereas 91% of women aged 50–74 years will undergo biennial mammography [ 105 ]. The primary endpoints are non-inferiority to standard screening regarding the number of late-stage breast cancers diagnosed (>stage IIB), and rates of recall and breast biopsy. Secondary endpoints include the rate of stage IIB and interval cancers, recall rates, rates of DCIS diagnosis, rates of chemoprevention use, cancer incidence rate, PROMIS anxiety score and rates of systemic therapy use between arms. Importantly, the design is inherently adaptive so that risk assessment methodology and screening strategies are adjustable in line with future evidence under a ‘continuous improvement’ framework [ 18 ].
The population-based Tailored Breast Screening Trial (NCT02619123) was initiated in Italy in 2013, and is randomising pre-menopausal women aged 44 years and older to invitation to ‘tailored screening’ or an active comparator [ 107 ]. The target recruitment is 33,200 women and the estimated study completion date will be early 2022. In the tailored arm, those with BI-RADS grade C–D breast density receive annual mammogram invitations until age 50 years and then standard population screening; those with lower density breasts are invited 2-yearly until age 50 years and then standard population screening. In the active comparator arm, women are invited to annual mammography until age 50 years, followed by usual population screening. The primary outcome measures are the difference in cumulative interval cancers between arms (also by density group) and the cumulative incidence of >T2 or node-positive breast cancers by arms (also by density group). Secondary endpoints include a comparison of false-positive rates between arms, the cumulative incidence of all breast cancer cases and attendance to screening. However, the ramifications of this trial on clinical practice may be limited, due to the basis for stratification (dense versus non-dense breasts), the small divergences in screening strategy (annual or biennial mammography) and the short period in which the screening intensity will be altered.
The other key trial is My Personalized Breast Screening (NCT03672331); an international study seeking to recruit 85,000 women aged 40–70 years, in which screening strategy in the experimental arm will be dictated by risk assessment incorporating age, family history, previous benign breast disease, hormone/reproductive history and a polygenic risk score. Specifically, women with one or no first-degree relatives with breast or ovarian cancer will utilise the MammoRisk® model, otherwise, the Tyrer–Cuzick model will be used (see above). No data regarding the proprietary MammoRisk® algorithm structure itself or results of performance evaluation is accessible on the owning company’s website ( https://www.predilife.com/en/home-2/ , accessed 1 November 2020) or identifiable on Medline, although studies of acceptability/ease of software use are available [ 109 , 110 ]. In the comparator arm, women will be screened with mammography, tomosynthesis or MRI in accordance with extant national guidelines, whereas in the active arm, an estimated 5-year risk will inform mammography and/or tomosynthesis screening every 1 to 4 years (with or without ultrasound depending on breast density). The trial has a non-inferiority design and the primary outcome is the incidence of stage >II breast cancers for the risk-stratified arm. Secondary outcome measures include a superiority analysis regarding the incidence rate of stage >II cancers, rates of false positives and benign biopsies, subject anxiety, health-related quality of life according to the EQ-5D and cumulative breast cancer diagnosis rates.
Overall, three key randomised trials are underway to assess the outcomes associated with different screening intervals, starting age, or imaging modalities based on individualised risk assessment, although there is diversity in the robustness of the risk assessment mechanism. The initial results from these trials are likely to emerge within the next 3 years, and it is notable that the largest and most comprehensive is adaptable [ 105 ], in that newer methods of risk assessment or amended screening strategies can be incorporated should novel evidence emerge.
Health economic evaluations of stratified screening
Economic simulations of risk-stratified breast screening have modelled the clinical outcomes and cost-effectiveness of a range of scenarios in health systems such as the United Kingdom’s NHS Breast Screening Programme [ 16 , 111 ], the United States [ 112 , 113 ], Germany [ 114 , 115 ], the Netherlands [ 116 ] and China [ 117 ]. Models have evaluated the stratification of screening intensity based on classical risk factors such as family history, class of breast density, age or relative risk based on polygenic risk scores. Across models, analytical approaches have been compared, e.g. relative numbers of breast cancer deaths avoided, rates of overdiagnosis, incremental costs or incremental quality-adjusted life-years and incremental cost-effectiveness ratios.
Whilst it appears that such modelling does lend support to the general concept of altering screening practices on the basis of risk to strike a more favourable balance of benefits (reduction in breast cancer deaths) and harms (overdiagnosis and unnecessary treatment) or to formulate a more cost-effective approach, a cohesive narrative regarding a particular algorithm-informed strategy is difficult to synthesise. Table 4 summarises the approaches and key results from such studies. These existing studies may diverge from real-world practice in terms of the ascertainment of individual risk (in terms of nuance and approach) or may be limited by incomplete information on risk distributions in the target population.
More simplistic estimations of relative risk on a small number of covariates tend to be used as the stratification mechanism, such as ‘positive family history’ or breast density category, or use blunter approaches to ascertain ‘high-risk’ women, such as the relative risk of 2.0 or greater. Multivariable risk assessment seeks to offer more nuanced estimations of risk, which are not recapitulated in many studies. Modelling the implementation of a polygenic risk assessment to inform relative risk may be limited by the relatively small absolute contribution that genetic factors may play in the majority of women that do not have high- or medium-penetrant mutations. As some studies have highlighted, the lack of data on the true distribution of risk groups in the target population as a whole limits their assessment of the impact of risk-based screening practices in a health system.
The approaches to and thresholds used to risk-stratify screening appear to differ between studies. Some seek to find the economically optimal screening interval or starting age for screening in cohorts simulated as having set risk levels, or even have intensified screening with additional imaging modalities as one pathway in their models. Few compare existing age-based methods with risk-stratified approaches in the same target population, and fewer evaluate a more robust view of risk-adapted screening, namely not offering screening to those at the lowest risk. Furthermore, the direct comparator in some studies is ‘no screening’ rather than an evaluation of transitioning from age-based screening to truly risk-adapted screening.
Qualitative research
In order to accept screening strategies tailored to individual risk, women need to be able to access and comprehend accurate risk estimations [ 118 , 119 , 120 ], and indeed, many women are interested to understand and discuss their risk [ 90 , 101 , 102 , 121 , 122 ]. Risk communication and risk perception are multifaceted and complex [ 119 ], yet it is striking that as few as 10% of women have accurate perceptions of personal risk with an otherwise roughly even split between under- and over-estimators [ 123 , 124 ]. The variable use of absolute and relative risks can have major influences on screening intentions or in some cases be misleading [ 125 ].
A growing body of qualitative research has synthesised evidence from focus groups, semi-structured interviews and other methodologies regarding stakeholder views on the implementation and acceptability of risk-stratified screening [ 90 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 ]. Particularly key considerations are those pertaining to communication of risk and risk-based pathways across languages and cultural groups [ 127 ], socioeconomic groups and those that have lower engagement with preventive healthcare. Generally, perceptions of risk-based screening appear to be favourable, whether based on genetic risk [ 133 , 137 ] or other factors. Whilst it felt to be acceptable in principle by many women, the evidence base used to support these approaches needs clear articulation to secure buy-in [ 126 ] and reasons for heterogeneous policies for different groups need to be transparent [ 126 ]. Importantly, perceptions of risk stratification as a euphemism for service funding reductions may arise [ 128 ], and there should be cognisance of anxiety around self-directed risk assessment if used, such as via websites or apps [ 138 ] (albeit, at low levels) [ 134 ].
Genetic, pharmacological and environmental or lifestyle factors affect breast cancer risk, with heterogeneity encountered in terms of individual women’s risks and the risks posed by individual tumours. As one-size-fits-all approaches are increasingly disparaged in breast cancer treatment, detection and prevention under the auspices of ‘personalised healthcare’ [ 21 ], the use of age as the single precision factor for guiding early detection strategies in women may be over-reductionist [ 19 , 20 , 21 ]. Approximately one-quarter of all breast cancers are diagnosed in women ineligible for screening due to age, only around 30% of breast cancers in the United Kingdom are detected by the triennial UK screening programme [ 139 ] (43% of all breast cancers in the United States [ 140 ]), and current methods may predicate overdiagnosis [ 3 , 5 , 8 , 11 ]. In the United States, 73% of breast cancers in biennial screeners are detected by mammography, and 78% of breast cancers are detected by screening in annual screeners [ 141 ]. Approaches that tailor screening intensity and personalise modalities to those that stand most to gain and minimise unnecessary interventions in those with little to gain require robust evaluation.
It is relevant to distinguish between the use of genetic, lifestyle and other factors in tools for long-term risk prediction to guide imaging strategies over a woman’s ‘screening lifetime’ and estimating the risk of an underlying cancer being present at the time of screening. Compared to settings such as lung cancer screening, where smoking history has the predominant effect on decision-making and may approach immediate diagnostic relevance, the long-term prediction of breast cancer relies on appropriate multi-factorial assessment using data points that have ‘weaker’ effects on risk.
Approaches to risk-stratified screening that have been explored include altering screening intensity, screening starting age or screening technology used and therefore a single consensus definition has not emerged, nor on the optimal form risk-stratified screening could take. One concern is that a paradigm withholding access to screening with a sensitive imaging modality in those deemed ‘low risk’ by a less sensitive predictive model may be inappropriate. As such, there must be judicious consideration of whether a ‘screen-only high-risk’ model is ethically, economically and clinically appropriate in comparison to other models such as the current effective approach, or those that seek to deliver screening that is ‘tailored according to risk’, where risk assessment influences the factors mentioned above without removing women from the screening pool. It is not yet clear which if any of the currently available risk models or risk-based strategies deliver on this concept—ongoing trials and other continued progress in optimising individualised risk estimation and screening strategy should begin to deliver clinically informative answers in the coming years [ 105 , 106 , 107 ]. Further, individualised risk estimation may not only be useful for stratifying screening strategy to reduce harms but to also reduce the substantial public health burden of breast cancer, potentially through identifying women at previously unknown high risk suitable for prevention therapy (e.g. Anastrozole or Tamoxifen).
Alongside the elucidation of the optimal degree of nuance for breast screening pathways, it would be essential to explore risk communication strategies, and continually monitor such programs’ effectiveness both clinically and economically. Another avenue for future work will be further assessment of stated preferences, such as through discrete choice experiments [ 142 , 143 ] or contingent valuation studies, and the trade-offs between benefits, risks and costs in making such preferences in a stratified programme.
Concerns have been raised that risk-stratified screening poses the danger that many breast cancers may be ‘missed’ by not screening women believed to be at low risk [ 84 , 144 ]. However, many such studies ignore the inherently multivariable nature of the best performing currently available risk models [ 30 , 47 , 50 ], compare current age-based screening against a straw man of blunt risk estimation that is not advocated, and are not powered to identify the effects on important outcomes such as the proportion of late-stage cancer detected, or cancer survival. Stratification may lead to changes in imaging strategy, such as increased use of supplemental MRI, which randomised trial evidence suggests reduces rates of interval cancers in women with extremely dense breast tissue [ 145 ]. Recent evidence of supplemental abbreviated MRI in women at average risk with dense breasts and negative digital tomosynthesis results appears to increase the prevalent cancer detection rate (up to 27.4 per 1000 women), but the survival benefits are yet to be quantified [ 146 ].
Overdiagnosis and overtreatment have been widely acknowledged in prostate cancer screening for years [ 147 , 148 , 149 , 150 ], yet a progression towards risk-stratification of screening itself or risk-guided management of detected neoplasms is far more mature than in the breast cancer field [ 149 , 151 ]. The debate around differing estimates of screening mammography’s benefits and harms has become increasingly polarised over recent decades [ 4 , 18 , 152 , 153 , 154 , 155 , 156 , 157 , 158 ], with consistent disagreement over the interpretation of decades-old trials, the reliability of specific randomised studies, statistical approaches used to interpret them or epidemiological studies [ 11 , 158 , 159 , 160 ]. However, whilst the clearly prevailing consensus is that screening mammography reduces breast cancer mortality, whether this can be further improved is a worthy avenue to explore. Recently, trials have been designed to provide evidence to inform this. Should their results be positive, they must be followed by careful consideration of whether the ‘new normal’ would be acceptable to healthcare systems, policymakers, clinicians and members of the public.
Data availability
No original data were generated for this review. The literature search strategy is provided.
Balmana J, Diez O, Rubio IT, Cardoso F, Group EGW. BRCA in breast cancer: ESMO Clinical Practice Guidelines. Ann Oncol. 2011;22 Suppl 6:vi31–34.
Article PubMed Google Scholar
Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, et al. Breast cancer. Nat Rev Dis Prim. 2019;5:66.
Marmot MG, Altman DG, Cameron DA, Dewar JA, Thompson SG, Wilcox M. The benefits and harms of breast cancer screening: an independent review. Br J Cancer. 2013;108:2205–40.
Article CAS PubMed PubMed Central Google Scholar
Gotzsche PC. Mammography screening is harmful and should be abandoned. J R Soc Med. 2015;108:341–5.
Article PubMed PubMed Central Google Scholar
Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ. 2014;348:g366.
Bleyer A, Welch HG. Effect of three decades of screening mammography on breast-cancer incidence. N. Engl J Med. 2012;367:1998–2005.
Article CAS PubMed Google Scholar
Kalager M, Loberg M, Bretthauer M, Adami HO. Comparative analysis of breast cancer mortality following mammography screening in Denmark and Norway. Ann Oncol. 2014;25:1137–43.
Loberg M, Lousdal ML, Bretthauer M, Kalager M. Benefits and harms of mammography screening. Breast Cancer Res. 2015;17:63.
Kalager M, Zelen M, Langmark F, Adami HO. Effect of screening mammography on breast-cancer mortality in Norway. N. Engl J Med. 2010;363:1203–10.
Jorgensen KJ, Gotzsche PC, Kalager M, Zahl PH. Breast Cancer Screening in Denmark: a cohort study of tumor size and overdiagnosis. Ann Intern Med. 2017;166:313–23.
Autier P, Boniol M. Mammography screening: a major issue in medicine. Eur J Cancer. 2018;90:34–62.
Paci E, Group EW. Summary of the evidence of breast cancer service screening outcomes in Europe and first estimate of the benefit and harm balance sheet. J Med Screen. 2012;19 Suppl 1:5–13.
Njor SH, Garne JP, Lynge E. Over-diagnosis estimate from The Independent UK Panel on Breast Cancer Screening is based on unsuitable data. J Med Screen. 2013;20:104–5.
Baker SG, Prorok PC. Breast cancer overdiagnosis in stop-screen trials: more uncertainty than previously reported. J Med Screen. 2020. https://doi.org/10.1177/0969141320950784 .
Baum M. Should routine screening by mammography be replaced by a more selective service of risk assessment/risk management? Women’s Health. 2010;6:71–6.
PubMed Google Scholar
Pashayan N, Morris S, Gilbert FJ, Pharoah PDP. Cost-effectiveness and benefit-to-harm ratio of risk-stratified screening for breast cancer: a Life-Table Model. JAMA Oncol. 2018;4:1504–10.
Onega T, Beaber EF, Sprague BL, Barlow WE, Haas JS, Tosteson AN, et al. Breast cancer screening in an era of personalized regimens: a conceptual model and National Cancer Institute initiative for risk-based and preference-based approaches at a population level. Cancer. 2014;120:2955–64.
Esserman LJ, Study W, Athena I. The WISDOM Study: breaking the deadlock in the breast cancer screening debate. NPJ Breast Cancer. 2017;3:34.
Bitencourt AG, Rossi Saccarelli C, Kuhl C, Morris EA. Breast cancer screening in average-risk women: towards personalized screening. Br J Radiol. 2019;92:20190660.
Kerlikowske K, O’Kane ME, Esserman LJ. Fifty years of age-based screening: time for a new risk-based screening approach. Evid Based Med. 2014;19:183.
Pashayan N, Antoniou AC, Ivanus U, Esserman LJ, Easton DF, French D, et al. Personalized early detection and prevention of breast cancer: ENVISION consensus statement. Nat Rev Clin Oncol. 2020;17:687–705.
Harkness EF, Astley SM, Evans DG. Risk-based breast cancer screening strategies in women. Best Pract Res Clin Obstet Gynaecol. 2020;65:3–17.
Allweis, TM, Hermann N, Berenstein-Molho R, Guindy M. Personalized screening for breast cancer: rationale, present practices, and future directions. Ann Surg Oncol. 2021. https://doi.org/10.1245/s10434-020-09426-1 .
Ryan R. Cochrane Consumers and Communication Review Group. Data synthesis and analysis. 2013. http://cccrg.cochrane.org .
Engmann NJ, Golmakani MK, Miglioretti DL, Sprague BL, Kerlikowske K, Breast Cancer Surveillance, C. Population-attributable risk proportion of clinical risk factors for breast cancer. JAMA Oncol. 2017;3:1228–36.
Amadou A, Hainaut P, Romieu I. Role of obesity in the risk of breast cancer: lessons from anthropometry. J Oncol. 2013;2013:906495.
Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67:378–97.
Ueda K, Tsukuma H, Tanaka H, Ajiki W, Oshima A. Estimation of individualized probabilities of developing breast cancer for Japanese women. Breast Cancer. 2003;10:54–62.
Tyrer J, Duffy SW, Cuzick J. A breast cancer prediction model incorporating familial and personal risk factors. Stat Med. 2004;23:1111–30.
van Veen EM, Brentnall AR, Byers H, Harkness EF, Astley SM, Sampson S, et al. Use of single-nucleotide polymorphisms and mammographic density plus classic risk factors for breast cancer risk prediction. JAMA Oncol. 2018;4:476–82.
Antoniou AC, Cunningham AP, Peto J, Evans DG, Lalloo F, Narod SA, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457–66.
Antoniou AC, Hardy R, Walker L, Evans DG, Shenton A, Eeles R, et al. Predicting the likelihood of carrying a BRCA1 or BRCA2 mutation: validation of BOADICEA, BRCAPRO, IBIS, Myriad and the Manchester scoring system using data from UK genetics clinics. J Med Genet. 2008;45:425–31.
Antoniou AC, Pharoah PP, Smith P, Easton DF. The BOADICEA model of genetic susceptibility to breast and ovarian cancer. Br J Cancer. 2004;91:1580–90.
Antoniou AC, Durocher F, Smith P, Simard J, Easton DF, members, I. B. P. BRCA1 and BRCA2 mutation predictions using the BOADICEA and BRCAPRO models and penetrance estimation in high-risk French-Canadian families. Breast Cancer Res. 2006;8:R3.
Eccles SA, Aboagye EO, Ali S, Anderson AS, Armes J, Berditchevski F, et al. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. 2013;15:R92.
Yanes T, Young MA, Meiser B, James PA. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res. 2020;22:21.
Michailidou K, Hall P, Gonzalez-Neira A, Ghoussaini M, Dennis J, Milne RL, et al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk. Nat Genet. 2013;45:353–61. 361e351–52
Michailidou K, Beesley J, Lindstrom S, Canisius S, Dennis J, Lush MJ, et al. Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nat Genet. 2015;47:373–80.
Mavaddat N, Pharoah PD, Michailidou K, Tyrer J, Brook MN, Bolla MK, et al. Prediction of breast cancer risk based on profiling with common genetic variants. J Natl Cancer Inst. 2015;107:djv036.
Mavaddat N, Michailidou K, Dennis J, Lush M, Fachal L, Lee A, et al. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Hum Genet. 2019;104:21–34.
Winkel RR, von Euler-Chelpin M, Nielsen M, Petersen K, Lillholm M, Nielsen MB, et al. Mammographic density and structural features can individually and jointly contribute to breast cancer risk assessment in mammography screening: a case-control study. BMC Cancer. 2016;16:414.
Sprague BL, Conant EF, Onega T, Garcia MP, Beaber EF, Herschorn SD, et al. Variation in mammographic breast density assessments among radiologists in clinical practice: a multicenter observational study. Ann Intern Med. 2016;165:457–64.
Arieno A, Chan A, Destounis SV. A review of the role of augmented intelligence in breast imaging: from automated breast density assessment to risk stratification. Am J Roentgenol. 2019;212:259–70.
Article Google Scholar
Ekpo EU, Mello-Thoms C, Rickard M, Brennan PC, McEntee MF. Breast density (BD) assessment with digital breast tomosynthesis (DBT): agreement between Quantra and 5th edition BI-RADS((R)). Breast. 2016;30:185–90.
Ekpo EU, McEntee MF. Measurement of breast density with digital breast tomosynthesis-a systematic review. Br J Radiol. 2014;87:20140460.
Magny SJ, Shikhman R, Keppke AL. Breast imaging reporting and data system. In: StatPearls. StatPearls Publishing: Treasure Island; 2020.
Brentnall AR, Cuzick J, Buist DSM, Bowles EJA. Long-term accuracy of breast cancer risk assessment combining classic risk factors and breast density. JAMA Oncol. 2018;4:e180174.
Vachon CM, Pankratz VS, Scott CG, Haeberle L, Ziv E, Jensen MR, et al. The contributions of breast density and common genetic variation to breast cancer risk. J Natl Cancer Inst. 2015;107:dju397.
Tice JA, Cummings SR, Smith-Bindman R, Ichikawa L, Barlow WE, Kerlikowske K. Using clinical factors and mammographic breast density to estimate breast cancer risk: development and validation of a new predictive model. Ann Intern Med. 2008;148:337–47.
Hippisley-Cox J, Coupland C. Development and validation of risk prediction algorithms to estimate future risk of common cancers in men and women: prospective cohort study. BMJ Open. 2015;5:e007825.
Ming C, Viassolo V, Probst-Hensch N, Dinov ID, Chappuis PO, Katapodi MC. Machine learning-based lifetime breast cancer risk reclassification compared with the BOADICEA model: impact on screening recommendations. Br J Cancer. 2020;123:860–7.
Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD). Ann Intern Med. 2015;162:735–6.
Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–73.
Steyerberg EW, Harrell FE Jr. Prediction models need appropriate internal, internal-external, and external validation. J Clin Epidemiol. 2016;69:245–7.
Austin PC, van Klaveren D, Vergouwe Y, Nieboer D, Lee DS, Steyerberg EW. Validation of prediction models: examining temporal and geographic stability of baseline risk and estimated covariate effects. Diagn Progn Res. 2017;1:12.
Steyerberg EW, Vergouwe Y. Towards better clinical prediction models: seven steps for development and an ABCD for validation. Eur Heart J. 2014;35:1925–31.
Van Calster B, McLernon DJ, van Smeden M, Wynants L, Steyerberg EW, Topic Group ‘Evaluating diagnostic, t. et al. Calibration: the Achilles heel of predictive analytics. BMC Med. 2019;17:230.
Vickers AJ, Van Calster B, Steyerberg EW. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ. 2016;352:i6.
Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128–38.
Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115:928–35.
Royston P, Sauerbrei W. A new measure of prognostic separation in survival data. Stat Med. 2004;23:723–48.
Wynants L, Riley RD, Timmerman D, Van Calster B. Random-effects meta-analysis of the clinical utility of tests and prediction models. Stat Med. 2018;37:2034–52.
Riley RD, Ensor J, Snell KI, Debray TP, Altman DG, Moons KG, et al. External validation of clinical prediction models using big datasets from e-health records or IPD meta-analysis: opportunities and challenges. BMJ. 2016;353:i3140.
Louro J, Posso M, Hilton Boon M, Roman M, Domingo L, Castells X, et al. A systematic review and quality assessment of individualised breast cancer risk prediction models. Br J Cancer. 2019;121:76–85.
Jaime Caro J, Eddy DM, Kan H, Kaltz C, Patel B, Eldessouki R, et al. Questionnaire to assess relevance and credibility of modeling studies for informing health care decision making: an ISPOR-AMCP-NPC Good Practice Task Force report. Value Health. 2014;17:174–82.
Moons KGM, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med. 2019;170:W1–W33.
Anothaisintawee T, Teerawattananon Y, Wiratkapun C, Srinakarin J, Woodtichartpreecha P, Hirunpat S, et al. Development and validation of a breast cancer risk prediction model for Thai women: a cross-sectional study. Asian Pac J Cancer Prev. 2014;15:6811–7.
Brentnall AR, Harkness EF, Astley SM, Donnelly LS, Stavrinos P, Sampson S, et al. Mammographic density adds accuracy to both the Tyrer-Cuzick and Gail breast cancer risk models in a prospective UK screening cohort. Breast Cancer Res. 2015;17:147.
Vilmun BM, Vejborg I, Lynge E, Lillholm M, Nielsen M, Nielsen MB, et al. Impact of adding breast density to breast cancer risk models: a systematic review. Eur J Radio. 2020;127:109019.
Van Calster B, Nieboer D, Vergouwe Y, De Cock B, Pencina MJ, Steyerberg EW. A calibration hierarchy for risk models was defined: from utopia to empirical data. J Clin Epidemiol. 2016;74:167–76.
Terry MB, Liao Y, Whittemore AS, Leoce N, Buchsbaum R, Zeinomar N, et al. 10-year performance of four models of breast cancer risk: a validation study. Lancet Oncol. 2019;20:504–17.
Christodoulou E, Ma J, Collins GS, Steyerberg EW, Verbakel JY, Van Calster B. A systematic review shows no performance benefit of machine learning over logistic regression for clinical prediction models. J Clin Epidemiol. 2019;110:12–22.
Haibe-Kains B, Adam GA, Hosny A, Khodakarami F, Massive Analysis Quality Control Society Board of, D., Waldron L, et al. Transparency and reproducibility in artificial intelligence. Nature. 2020;586:E14–6.
Varma S, Simon R. Bias in error estimation when using cross-validation for model selection. BMC Bioinform. 2006;7:91.
Vabalas A, Gowen E, Poliakoff E, Casson AJ. Machine learning algorithm validation with a limited sample size. PLoS ONE. 2019;14:e0224365.
Austin PC, van Klaveren D, Vergouwe Y, Nieboer D, Lee DS, Steyerberg EW. Geographic and temporal validity of prediction models: different approaches were useful to examine model performance. J Clin Epidemiol. 2016;79:76–85.
Pal Choudhury P, Wilcox AN, Brook MN, Zhang Y, Ahearn T, Orr N, et al. Comparative validation of breast cancer risk prediction models and projections for future risk stratification. J Natl Cancer Inst. 2020;112:278–85.
Taplin SH, Thompson RS, Schnitzer F, Anderman C, Immanuel V. Revisions in the risk-based Breast Cancer Screening Program at Group Health Cooperative. Cancer. 1990;66:812–8.
Yen AM, Tsau HS, Fann JC, Chen SL, Chiu SY, Lee YC, et al. Population-based breast cancer screening with risk-based and universal mammography screening compared with clinical breast examination: a propensity score analysis of 1 429 890 Taiwanese women. JAMA Oncol. 2016;2:915–21.
Liu FC, Lin HT, Kuo CF, See LC, Chiou MJ, Yu HP. Epidemiology and survival outcome of breast cancer in a nationwide study. Oncotarget. 2017;8:16939–50.
Mukama T, Kharazmi E, Xing X, Sundquist K, Sundquist J, Brenner H, et al. Risk-adapted starting age of screening for relatives of patients with breast cancer. JAMA Oncol. 2019;6:68–74.
Article PubMed Central Google Scholar
Mukama T, Fallah M, Tian Y, Sundquist K, Sundquist J, Brenner H, et al. Risk-tailored starting age of breast cancer screening based on women’s reproductive profile: a nationwide cohort study. Eur J Cancer. 2020;124:207–13.
Duffy SW, Vulkan D, Cuckle H, Parmar D, Sheikh S, Smith RA, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21:1165–72.
Feig SA. Personalized screening for breast cancer: a wolf in sheep’s clothing? Am J Roentgenol. 2015;205:1365–71.
Joe BN, Hayward JH. More lives risked with risk-based versus age-based breast cancer screening. Radiology. 2019;292:329–30.
Neal CH, Rahman WT, Joe AI, Noroozian M, Pinsky RW, Helvie MA. Harms of restrictive risk-based mammographic breast cancer screening. Am J Roentgenol. 2018;210:228–34.
Burnside ES, Trentham-Dietz A, Shafer CM, Hampton JM, Alagoz O, Cox JR, et al. Age-based versus risk-based mammography screening in women 40-49 years old: a cross-sectional study. Radiology. 2019;292:321–8.
Lee CS, Ashih H, Sengupta D, Sickles EA, Zuley M, Pisano E. Risk-based screening mammography for women aged <40: outcomes from the national mammography database. J Am Coll Radiol. 2020;17:368–76.
Price ER, Keedy AW, Gidwaney R, Sickles EA, Joe BN. The potential impact of risk-based screening mammography in women 40-49 years old. Am J Roentgenol. 2015;205:1360–4.
Rainey L, van der Waal D, Broeders MJM. Dutch women’s intended participation in a risk-based breast cancer screening and prevention programme: a survey study identifying preferences, facilitators and barriers. BMC Cancer. 2020;20:965.
Rainey L, van der Waal D, Jervaeus A, Donnelly LS, Evans DG, Hammarstrom M, et al. European women’s perceptions of the implementation and organisation of risk-based breast cancer screening and prevention: a qualitative study. BMC Cancer. 2020;20:247.
Gabrielson M, Eriksson M, Hammarstrom M, Borgquist S, Leifland K, Czene K, et al. Cohort profile: the Karolinska Mammography Project for Risk Prediction of Breast Cancer (KARMA). Int J Epidemiol. 2017;46:1740–41.
Eriksson M, Czene K, Pawitan Y, Leifland K, Darabi H, Hall P. A clinical model for identifying the short-term risk of breast cancer. Breast Cancer Res. 2017;19:29.
Vachon CM, Scott CG, Tamimi RM, Thompson DJ, Fasching PA, Stone J, et al. Joint association of mammographic density adjusted for age and body mass index and polygenic risk score with breast cancer risk. Breast Cancer Res. 2019;21:68.
Ferreira MA, Gamazon ER, Al-Ejeh F, Aittomaki K, Andrulis IL, Anton-Culver H, et al. Genome-wide association and transcriptome studies identify target genes and risk loci for breast cancer. Nat Commun. 2019;10:1741.
Escala-Garcia M, Guo Q, Dork T, Canisius S, Keeman R, Dennis J, et al. Genome-wide association study of germline variants and breast cancer-specific mortality. Br J Cancer. 2019;120:647–57.
Garcia-Closas M, Couch FJ, Lindstrom S, Michailidou K, Schmidt MK, Brook MN, et al. Genome-wide association studies identify four ER negative-specific breast cancer risk loci. Nat Genet. 2013;45:392–8. 398e391–92
Garcia-Closas M, Gail MH, Kelsey KT, Ziegler RG. Searching for blood DNA methylation markers of breast cancer risk and early detection. J Natl Cancer Inst. 2013;105:678–80.
Evans DG, Astley S, Stavrinos P, Harkness E, Donnelly LS, Dawe S, et al. Improvement in risk prediction, early detection and prevention of breast cancer in the NHS Breast Screening Programme and family history clinics: a dual cohort study. In: Improvement in risk prediction, early detection and prevention of breast cancer in the NHS Breast Screening Programme and family history clinics: a dual cohort study. NIHR Journals Library; 2016. https://doi.org/10.3310/pgfar04110 .
Evans DGR, Harkness EF, Brentnall AR, van Veen EM, Astley SM, Byers H, et al. Breast cancer pathology and stage are better predicted by risk stratification models that include mammographic density and common genetic variants. Breast Cancer Res Treat. 2019;176:141–8.
Evans DG, Donnelly LS, Harkness EF, Astley SM, Stavrinos P, Dawe S, et al. Breast cancer risk feedback to women in the UK NHS breast screening population. Br J Cancer. 2016;114:1045–52.
Evans DG, Howell A. Can the breast screening appointment be used to provide risk assessment and prevention advice? Breast Cancer Res. 2015;17:84.
French DP, Southworth J, Howell A, Harvie M, Stavrinos P, Watterson D, et al. Psychological impact of providing women with personalised 10-year breast cancer risk estimates. Br J Cancer. 2018;118:1648–57.
Howell A, Astley S, Warwick J, Stavrinos P, Sahin S, Ingham S, et al. Prevention of breast cancer in the context of a national breast screening programme. J Intern Med. 2012;271:321–30.
Shieh Y, Eklund M, Madlensky L, Sawyer SD, Thompson CK, Stover Fiscalini A, et al. Breast Cancer screening in the precision medicine era: risk-based screening in a population-based trial. J Natl Cancer Inst. 2017;109:djw290.
Giordano L, Gallo F, Petracci E, Chiorino G, Segnan N. The ANDROMEDA prospective cohort study: predictive value of combined criteria to tailor breast cancer screening and new opportunities from circulating markers: study protocol. BMC Cancer. 2017;17:785.
Paci E, Mantellini P, Giorgi Rossi P, Falini P, Puliti D. Tailored Breast Screening Trial (TBST). Epidemiol Prev. 2013;37:317–27.
French DP, Astley S, Brentnall AR, Cuzick J, Dobrashian R, Duffy SW, et al. What are the benefits and harms of risk stratified screening as part of the NHS breast screening Programme? Study protocol for a multi-site non-randomised comparison of BC-predict versus usual screening (NCT04359420). BMC Cancer. 2020;20:570.
Uzan C, Ndiaye-Gueye D, Nikpayam M, Oueld Es Cheikh E, Lebegue G, Canlorbe G, et al. First results of a breast cancer risk assessment and management consultation. Bull Cancer. 2020;107:972–81.
Weigert J, Cavanaugh N, Ju T. Evaluating mammographer acceptance of MammoRisk Software. Radio Technol. 2018;89:344–50.
Google Scholar
Gray E, Donten A, Karssemeijer N, van Gils C, Evans DG, Astley S, et al. Evaluation of a stratified National Breast Screening Program in the United Kingdom: an early model-based cost-effectiveness analysis. Value Health. 2017;20:1100–9.
Trentham-Dietz A, Kerlikowske K, Stout NK, Miglioretti DL, Schechter CB, Ergun MA, et al. Tailoring breast cancer screening intervals by breast density and risk for women aged 50 years or older: collaborative modeling of screening outcomes. Ann Intern Med. 2016;165:700–12.
Schousboe JT, Kerlikowske K, Loh A, Cummings SR. Personalizing mammography by breast density and other risk factors for breast cancer: analysis of health benefits and cost-effectiveness. Ann Intern Med. 2011;155:10–20.
Arnold M, Pfeifer K, Quante AS. Is risk-stratified breast cancer screening economically efficient in Germany? PLoS ONE. 2019;14:e0217213.
Arnold M, Quante AS. Personalized mammography screening and screening adherence-a simulation and economic evaluation. Value Health. 2018;21:799–808.
Sankatsing VDV, van Ravesteyn NT, Heijnsdijk EAM, Broeders MJM, de Koning HJ. Risk stratification in breast cancer screening: cost-effectiveness and harm-benefit ratios for low-risk and high-risk women. Int J Cancer. 2020;147:3059–67.
Sun L, Legood R, Sadique Z, Dos-Santos-Silva I, Yang L. Cost-effectiveness of risk-based breast cancer screening programme, China. Bull World Health Organ. 2018;96:568–77.
Fagerlin A, Zikmund-Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103:1436–43.
de Jonge ET, Vlasselaer J, Van de Putte G, Schobbens JC. The construct of breast cancer risk perception: need for a better risk communication? Facts Views Vis Obgyn. 2009;1:122–9.
PubMed PubMed Central Google Scholar
Edwards AG, Naik G, Ahmed H, Elwyn GJ, Pickles T, Hood K, et al. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database Syst Rev. 2013;2013:Cd001865.
PubMed Central Google Scholar
Evans DG, Brentnall AR, Harvie M, Dawe S, Sergeant JC, Stavrinos P, et al. Breast cancer risk in young women in the national breast screening programme: implications for applying NICE guidelines for additional screening and chemoprevention. Cancer Prev Res. 2014;7:993–1001.
Yanes T, Kaur R, Meiser B, Scheepers-Joynt M, McInerny S, Barlow-Stewart K, et al. Women’s responses and understanding of polygenic breast cancer risk information. Fam Cancer. 2020;19:297–306.
Printz C. Most women have an inaccurate perception of their breast cancer risk. Cancer. 2014;120:314–5.
Abittan B, Pachtman S, Herman S, Indelicato J, Herman J. Perception of breast cancer risk in over 11,000 patients during routine mammography exam. J Cancer Educ. 2020;35:782–7.
Gigerenzer G. Breast cancer screening pamphlets mislead women. BMJ. 2014;348:g2636.
McWilliams L, Woof VG, Donnelly LS, Howell A, Evans DG, French DP. Risk stratified breast cancer screening: UK healthcare policy decision-making stakeholders’ views on a low-risk breast screening pathway. BMC Cancer. 2020;20:680.
Woof VG, Ruane H, French DP, Ulph F, Qureshi N, Khan N, et al. The introduction of risk stratified screening into the NHS breast screening Programme: views from British-Pakistani women. BMC Cancer. 2020;20:452.
He X, Schifferdecker KE, Ozanne EM, Tosteson ANA, Woloshin S, Schwartz LM. How do women view risk-based mammography screening? A qualitative study. J Gen Intern Med. 2018;33:1905–12.
Schifferdecker KE, Tosteson ANA, Kaplan C, Kerlikowske K, Buist DSM, Henderson LM, et al. Knowledge and perception of breast density, screening mammography, and supplemental screening: in search of “Informed”. J Gen Intern Med. 2020;35:1654–60.
Ghanouni A, Sanderson SC, Pashayan N, Renzi C, von Wagner C, Waller J. Attitudes towards risk-stratified breast cancer screening among women in England: a cross-sectional survey. J Med Screen. 2020;27:138–45.
Ghanouni A, Waller J, Stoffel ST, Vlaev I, von Wagner C. Acceptability of risk-stratified breast screening: effect of the order of presenting risk and benefit information. J Med Screen. 2020;27:52–6.
Lippey J, Keogh LA, Mann GB, Campbell IG, Forrest LE. “A Natural Progression”: Australian women’s attitudes about an individualized Breast Screening Model. Cancer Prev Res. 2019;12:383–90.
Meisel SF, Pashayan N, Rahman B, Side L, Fraser L, Gessler S, et al. Adjusting the frequency of mammography screening on the basis of genetic risk: Attitudes among women in the UK. Breast. 2015;24:237–41.
van Erkelens A, Sie AS, Manders P, Visser A, Duijm LE, Mann RM, et al. Online self-test identifies women at high familial breast cancer risk in population-based breast cancer screening without inducing anxiety or distress. Eur J Cancer. 2017;78:45–52.
Fürst N, Kiechle M, Strahwald B, Quante AS. Mammography Screening 2.0 - How can risk-adapted screening be implemented in clinical practice?: results of a focus group discussion with experts in the RISIKOLOTSE.DE Project. Geburtshilfe Frauenheilkd. 2018;78:506–11.
Rainey L, van der Waal D, Donnelly LS, Evans DG, Wengström Y, Broeders M. Women’s decision-making regarding risk-stratified breast cancer screening and prevention from the perspective of international healthcare professionals. PLoS ONE. 2018;13:e0197772.
Wong XY, Chong KJ, van Til JA, Wee HL. A qualitative study on Singaporean women’s views towards breast cancer screening and Single Nucleotide Polymorphisms (SNPs) gene testing to guide personalised screening strategies. BMC Cancer. 2017;17:776.
Elkin EB, Pocus VH, Mushlin AI, Cigler T, Atoria CL, Polaneczky MM. Facilitating informed decisions about breast cancer screening: development and evaluation of a web-based decision aid for women in their 40s. BMC Med Inform Decis Mak. 2017;17:29.
NICE. The NHS Breast Screening Programme. 2017. https://cks.nice.org.uk/topics/breast-screening/background-information/the-nhs-breast-screening-programme/ . Accessed 28th June 2021.
Roth MY, Elmore JG, Yi-Frazier JP, Reisch LM, Oster NV, Miglioretti DL. Self-detection remains a key method of breast cancer detection for U.S. women. J Women’s Health. 2011;20:1135–9.
Miglioretti DL, Zhu W, Kerlikowske K, Sprague BL, Onega T, Buist DS, et al. Breast tumor prognostic characteristics and biennial vs annual mammography, age, and menopausal status. JAMA Oncol. 2015;1:1069–77.
Vass CM, Rigby D, Payne K. Investigating the heterogeneity in women’s preferences for breast screening: does the communication of risk matter? Value Health. 2018;21:219–28.
Sicsic J, Pelletier-Fleury N, Moumjid N. Women’s benefits and harms trade-offs in breast cancer screening: results from a discrete-choice experiment. Value Health. 2018;21:78–88.
Carbillon L, Bricou A, Sellier N. Challenges, benefits, and harms of risk-based screening mammography in women 40-49 years old. Am J Roentgenol. 2016;206:W50.
Bakker MF, de Lange SV, Pijnappel RM, Mann RM, Peeters PHM, Monninkhof EM, et al. Supplemental MRI screening for women with extremely dense breast tissue. N. Engl J Med. 2019;381:2091–102.
Weinstein SP, Korhonen K, Cirelli C, Schnall MD, McDonald ES, Pantel AR, et al. Abbreviated breast magnetic resonance imaging for supplemental screening of women with dense breasts and average risk. J Clin Oncol. 2020;38:3874–82.
Pashayan N, Duffy SW, Chowdhury S, Dent T, Burton H, Neal DE, et al. Polygenic susceptibility to prostate and breast cancer: implications for personalised screening. Br J Cancer. 2011;104:1656–63.
Pashayan N, Duffy SW, Neal DE, Hamdy FC, Donovan JL, Martin RM, et al. Implications of polygenic risk-stratified screening for prostate cancer on overdiagnosis. Genet Med. 2015;17:789–95.
Vickers AJ. Redesigning prostate cancer screening strategies to reduce overdiagnosis. Clin Chem. 2019;65:39–41.
Vickers AJ, Sjoberg DD, Ulmert D, Vertosick E, Roobol MJ, Thompson I, et al. Empirical estimates of prostate cancer overdiagnosis by age and prostate-specific antigen. BMC Med. 2014;12:26.
Loeb S, Bjurlin MA, Nicholson J, Tammela TL, Penson DF, Carter HB, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65:1046–55.
Kopans DB. Arguments against mammography screening continue to be based on faulty science. Oncologist. 2014;19:107–12.
Kopans DB.The faulty analysis of breast cancer screening data.Acad Radiol. 2016;23:116–7.
Marmot MG. Sorting through the arguments on breast screening. JAMA. 2013;309:2553–4.
Bleyer A, Thomas CR Jr, Baines C, Miller AB. Flawed assumptions used to defend screening mammography. Cancer. 2015;121:320–1.
Gotzsche PC, Jorgensen KJ, Zahl PH. Breast screening: why estimates differ by a factor of 20-25. J Med Screen. 2010;17:158–9. author reply 159–60.
Jorgensen KJ, Gotzsche PC. Breast cancer screening: benefit or harm? JAMA. 2016;315:1402.
Jorgensen KJ, Keen JD, Zahl PH, Gotzsche PC. The Two-County breast screening trial cannot provide a reliable estimate of the effect of breast cancer screening. Radiology. 2012;262:729–30, author reply 730–21
Zahl PH, Jorgensen KJ, Gotzsche PC. Lead-time models should not be used to estimate overdiagnosis in cancer screening. J Gen Intern Med. 2014;29:1283–6.
Zahl PH, Jorgensen KJ, Gotzsche PC. Overestimated lead times in cancer screening has led to substantial underestimation of overdiagnosis. Br J Cancer. 2013;109:2014–9.
Department of Health & Social Care. NHS breast screening (BSP) programme. https://www.gov.uk/health-and-social-care/population-screening-programmes-breast . Accessed 27 Nov 2020.
US Preventive and Screening Task Force. Breast cancer: screening. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening . Accessed 27 Nov 2020.
Klarenbach S, Sims-Jones N, Lewin G, Singh H, Theriault G, Tonelli M, et al. Recommendations on screening for breast cancer in women aged 40-74 years who are not at increased risk for breast cancer. CMAJ. 2018;190:E1441–51.
Rijksintituut voor Volksgezondheid en Milieu. Bevolkingsonderzoek bortskanker. https://www.rivm.nl/bevolkingsonderzoek-borstkanker . Accessed 27 Nov 2020.
BreastScreen Australia. http://www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/breast-screening-1 . Accessed 27 Nov 2020.
National Health Commission Of The People’s Republic Of China. Chinese guidelines for diagnosis and treatment of breast cancer 2018 (English version). Chin J Cancer Res. 2019;31:259–77.
Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, et al. Projecting individualized probabilities of developing breast cancer for white females who are being examined annually. J Natl Cancer Inst. 1989;81:1879–86.
Gail MH, Costantino JP, Pee D, Bondy M, Newman L, Selvan M, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst. 2007;99:1782–92.
Tice JA, Cummings SR, Ziv E, Kerlikowske K. Mammographic breast density and the Gail model for breast cancer risk prediction in a screening population. Breast Cancer Res Treat. 2005;94:115–22.
Zhang X, Rice M, Tworoger SS, Rosner BA, Eliassen AH, Tamimi RM, et al. Addition of a polygenic risk score, mammographic density, and endogenous hormones to existing breast cancer risk prediction models: a nested case-control study. PLoS Med. 2018;15:e1002644.
Tice JA, Miglioretti DL, Li CS, Vachon CM, Gard CC, Kerlikowske K. Breast density and benign breast disease: risk assessment to identify women at high risk of breast cancer. J Clin Oncol. 2015;33:3137–43.
Rosner B, Colditz GA. Nurses’ health study: log-incidence mathematical model of breast cancer incidence. J Natl Cancer Inst. 1996;88:359–64.
Rosner B, Colditz GA, Iglehart JD, Hankinson SE. Risk prediction models with incomplete data with application to prediction of estrogen receptor-positive breast cancer: prospective data from the Nurses’ Health Study. Breast Cancer Res. 2008;10:R55.
Park B, Ma SH, Shin A, Chang MC, Choi JY, Kim S, et al. Korean risk assessment model for breast cancer risk prediction. PLoS ONE. 2013;8:e76736.
Abdolell M, Payne JI, Caines J, Tsuruda K, Barnes PJ, Talbot PJ, et al. Assessing breast cancer risk within the general screening population: developing a breast cancer risk model to identify higher risk women at mammographic screening. Eur Radiol. 2020;30:5417–26.
Wang Y, Gao Y, Battsend M, Chen K, Lu W, Wang Y. Development of a risk assessment tool for projecting individualized probabilities of developing breast cancer for Chinese women. Tumour Biol. 2014;35:10861–9.
Barlow WE, White E, Ballard-Barbash R, Vacek PM, Titus-Ernstoff L, Carney PA, et al. Prospective breast cancer risk prediction model for women undergoing screening mammography. J Natl Cancer Inst. 2006;98:1204–14.
van den Broek JJ, Schechter CB, van Ravesteyn NT, Janssens A, Wolfson MC, Trentham-Dietz A, et al. Personalizing breast cancer screening based on polygenic risk and family history. J Natl Cancer Inst. 2020. https://doi.org/10.1093/jnci/djaa127 .
Download references
Acknowledgements
We thank the anonymous reviewers whose comments on earlier versions of these manuscripts have helped materially improve this paper.
This work was supported by Cancer Research UK [C2195/A31310 to AKC, C8225/A21133 to DD, C49297/A27294 to GSC, C5255/A18085 to JH-C], the NIHR Biomedical Research Centre, Oxford [GSC], the NIHR (SP receives support as an NIHR Senior Investigator [NF-SI-0616-10103]) and the NIHR Applied Research Collaboration Oxford and Thames Valley (SP).
Author information
Authors and affiliations.
Cancer Research UK Oxford Centre, Department of Oncology, University of Oxford, Oxford, UK
Ash Kieran Clift
Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, UK
Ash Kieran Clift, Stavros Petrou & Julia Hippisley-Cox
Nuffield Department of Population Health, University of Oxford, Oxford, UK
David Dodwell
Department of Oncology, University of Oxford, Oxford, UK
Simon Lord & Sir Michael Brady
Centre for Statistics in Medicine, Nuffield Department of Orthopaedics, Rheumatology & Musculoskeletal Sciences, University of Oxford, Oxford, UK
Gary S. Collins
NIHR Oxford Biomedical Research Centre, John Radcliffe Hospital, Oxford, UK
You can also search for this author in PubMed Google Scholar
Contributions
AKC conceived the study, undertook the literature review and wrote the first draft of the manuscript. DD provided clinical expertise-based input throughout the study and critically reviewed the manuscript. SL provided clinical expertise-based input throughout the study and critically reviewed the manuscript. SP provided health economics expertise-based input throughout the study and critically reviewed the manuscript. MB provided imaging and technological expertise-based input throughout the study and critically reviewed the manuscript. GSC provided statistical expertise-based input throughout the study and critically reviewed the manuscript. JH-C helped conceive the study, provided epidemiology expertise-based input throughout the study and critically reviewed the manuscript.
Corresponding author
Correspondence to Ash Kieran Clift .
Ethics declarations
Competing interests.
JH-C is an unpaid director of QResearch (a not-for-profit organisation, which is a partnership between the University of Oxford and EMIS Health, which supply the QResearch database) and is a founder and shareholder of ClinRisk Ltd and was its medical director until 31 May 2019 (ClinRisk produces open and closed source software to implement clinical risk algorithms, including two breast cancer risk models implemented in the NHS into clinical computer systems (outside of this work)). All the remaining authors declare no competing interests.
Ethics approval and consent to participate
Not applicable—this review did not use original/clinical data from human participants or tissue.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Clift, A.K., Dodwell, D., Lord, S. et al. The current status of risk-stratified breast screening. Br J Cancer 126 , 533–550 (2022). https://doi.org/10.1038/s41416-021-01550-3
Download citation
Received : 22 January 2021
Revised : 25 August 2021
Accepted : 14 September 2021
Published : 26 October 2021
Issue Date : 09 March 2022
DOI : https://doi.org/10.1038/s41416-021-01550-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative

This article is cited by
A structured process for the validation of a decision-analytic model: application to a cost-effectiveness model for risk-stratified national breast screening.
- Stuart J. Wright
- Katherine Payne
Applied Health Economics and Health Policy (2024)
The risk-based breast screening (RIBBS) study protocol: a personalized screening model for young women
- Gisella Gennaro
- Lauro Bucchi
- Francesca Caumo
La radiologia medica (2024)
Rethinking screening mammography in Japan: next-generation breast cancer screening through breast awareness and supplemental ultrasonography
- Takayoshi Uematsu
Breast Cancer (2024)
Unlocking the complete blood count as a risk stratification tool for breast cancer using machine learning: a large scale retrospective study
- Daniella Castro Araujo
- Bruno Aragão Rocha
- Ismael Dale Cotrim Guerreiro da Silva
Scientific Reports (2024)
Understanding the contribution of lifestyle in breast cancer risk prediction: a systematic review of models applicable to Europe
- Elly Mertens
- Antonio Barrenechea-Pulache
- José L. Peñalvo
BMC Cancer (2023)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
A .gov website belongs to an official government organization in the United States.
A lock ( ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Risk Factors
- Breast Cancer Resources to Share
- What CDC Is Doing About Breast Cancer
- Advisory Committee on Breast Cancer in Young Women
- MMWR Appendix
- National Breast and Cervical Cancer Early Detection Program
- Bring Your Brave Campaign
Screening for Breast Cancer
- Breast cancer screening can help find breast cancer early, when it is easier to treat.
- The US Preventive Services Task Force recommends that women who are 40 to 74 years old and are at average risk for breast cancer get a mammogram every 2 years.
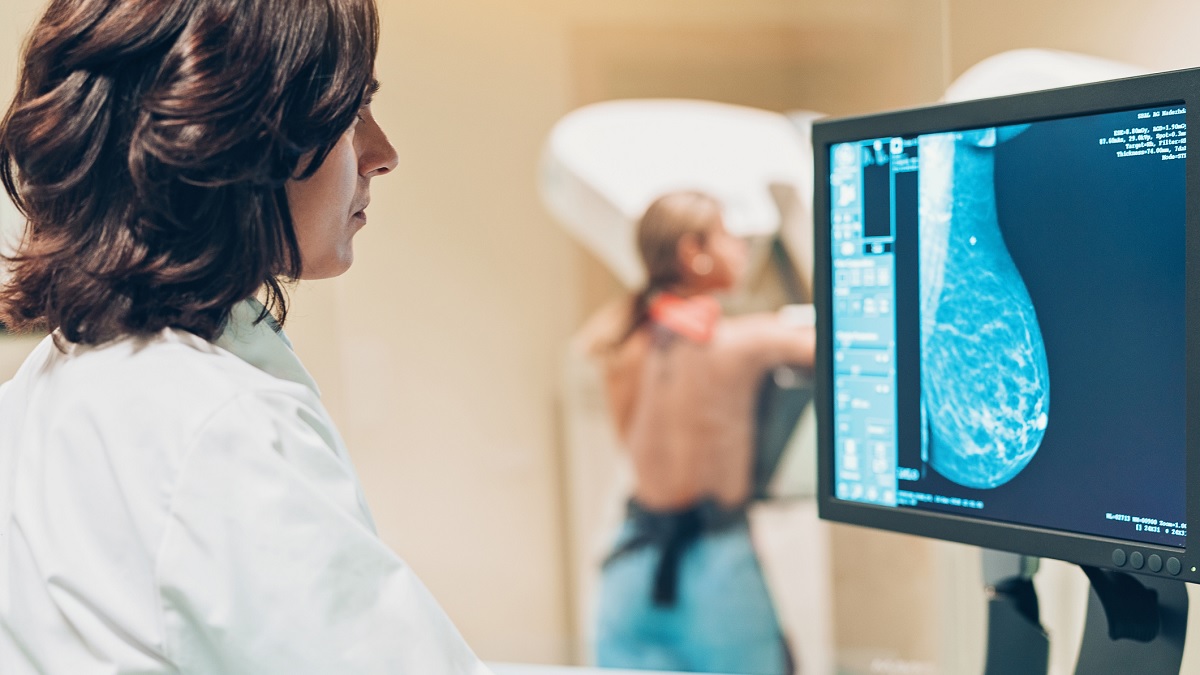
Breast cancer screening means checking a woman's breasts for cancer before there are signs or symptoms of the disease. All women need to be informed by their health care provider about the best screening options for them. When you are told about the benefits and risks of screening and decide with your health care provider whether screening is right for you—and if so, when to have it—this is called informed and shared decision-making.
Although breast cancer screening cannot prevent breast cancer, it can help find breast cancer early, when it is easier to treat. Talk to your doctor about which breast cancer screening tests are right for you, and when you should have them.
Screening recommendations
The US Preventive Services Task Force is an organization made up of doctors and disease experts who look at research on the best way to prevent diseases and make recommendations on how doctors can help patients avoid diseases or find them early.
The Task Force recommends that women who are 40 to 74 years old and are at average risk for breast cancer get a mammogram every 2 years. Women should weigh the benefits and risks of screening tests (see below).
When Should I Start Getting Mammograms?
CDC's Dr. Lisa Richardson talks about the best time for women to start getting mammograms in this video.
Types of tests
A mammogram is an x-ray of the breast. For many women, mammograms are the best way to find breast cancer early, when it is easier to treat and before it is big enough to feel or cause symptoms. Having regular mammograms can lower the risk of dying from breast cancer. At this time, a mammogram is the best way to find breast cancer for most women of screening age.
Breast magnetic resonance imaging (MRI)
A breast MRI uses magnets and radio waves to take pictures of the breast. Breast MRI is used along with mammograms to screen women who are at high risk for getting breast cancer. Because breast MRIs may appear abnormal even when there is no cancer, they are not used for women at average risk.
Other exams
- Clinical breast exam: A clinical breast exam is an examination by a doctor or nurse, who uses his or her hands to feel for lumps or other changes.
- Breast self-awareness: Being familiar with how your breasts look and feel can help you notice symptoms such as lumps, pain, or changes in size that may be of concern. These could include changes found during a breast self-exam. You should report any changes that you notice to your doctor or health care provider.
Having a clinical breast exam or doing a breast self-exam has not been found to lower the risk of dying from breast cancer.
Benefits and risks of screening
Every screening test has benefits and risks, which is why it's important to talk to your doctor before getting any screening test, like a mammogram.
Benefit of screening
The benefit of screening is finding cancer early, when it's easier to treat.
Risks of screening
Harms can include false positive test results, when a doctor sees something that looks like cancer but is not. This can lead to more tests, which can be expensive, invasive, and time-consuming, and may cause anxiety.
Tests also can lead to overdiagnosis, when doctors find a cancer that would not have gone on to cause symptoms or problems, or even may go away on its own. Treatment of these cancers is called overtreatment. Overtreatment can include treatments recommended for breast cancer, such as surgery or radiation therapy. These can cause unnecessary and unwanted side effects. Other potential harms from breast cancer screening include pain during procedures and radiation exposure from the mammogram test itself. While the amount of radiation in a mammogram is small, there may be risks with having repeated x-rays.
Mammograms may also miss some cancers, called false negative test results, which may delay finding a cancer and getting treatment.
Where can I go to get screened?
You can get screened for breast cancer at a clinic, hospital, or doctor's office. If you want to be screened for breast cancer, call your doctor's office. They can help you schedule an appointment.
Most health insurance plans are required to cover screening mammograms every 1 to 2 years for women beginning at age 40 with no out-of-pocket cost (like a co-pay, deductible, or co-insurance).
Find a mammography facility near you.
Are you worried about the cost?
Breast cancer.
Talk to your doctor about when to start and how often to get a mammogram.
For Everyone
Public health.

MUNEEZA KHAN, MD, AND ANNA CHOLLET, MD, MPH
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2021;103(1):33-41
Patient information: See related handout on mammogram screening for breast cancer , written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Breast cancer is the most common nonskin cancer in women and accounts for 30% of all new cancers in the United States. The highest incidence of breast cancer is in women 70 to 74 years of age. Numerous risk factors are associated with the development of breast cancer. A risk assessment tool can be used to determine individual risk and help guide screening decisions. The U.S. Preventive Services Task Force (USPSTF) and American Academy of Family Physicians (AAFP) recommend against teaching average-risk women to perform breast self-examinations. The USPSTF and AAFP recommend biennial screening mammography for average-risk women 50 to 74 years of age. However, there is no strong evidence supporting a net benefit of mammography screening in average-risk women 40 to 49 years of age; therefore, the USPSTF and AAFP recommend individualized decision-making in these women. For average-risk women 75 years and older, the USPSTF and AAFP conclude that there is insufficient evidence to recommend screening, but the American College of Obstetricians and Gynecologists and the American Cancer Society state that screening may continue depending on the woman's health status and life expectancy. Women at high risk of breast cancer may benefit from mammography starting at 30 years of age or earlier, with supplemental screening such as magnetic resonance imaging. Supplemental ultrasonography in women with dense breasts increases cancer detection but also false-positive results.
Breast cancer is the most common nonskin cancer in women and accounts for 30% of all new cancers in the United States. 1 From 2001 to 2016, more than 2.3 million women in the United States were diagnosed with breast cancer. 2 The incidence of breast cancer increases after 25 years of age, peaking between 70 and 74 years. 2 Approximately one in eight women will develop invasive breast cancer (12.8% lifetime risk). 1
WHAT'S NEW ON THIS TOPIC
Breast Cancer Screening
A 2016 meta-analysis calculated that per 10,000 women screened with mammography, three breast cancer deaths are avoided over 10 years in women 40 to 49 years of age, eight deaths are avoided in women 50 to 59 years, 21 deaths are avoided in women 60 to 69 years, and 13 deaths are avoided in women 70 to 74 years. [ corrected ]
One out of every eight women 40 to 49 years of age who has a screening mammogram will subsequently undergo additional imaging, and for every case of invasive breast cancer detected by screening mammography in this age group, 10 women will have had a biopsy.
In a large, multicenter trial, women with dense breasts and a negative standard mammogram result had two-year screening with MRI or standard mammography. The interval cancer rate was lower in the MRI group than in the mammography group; however, MRI had a high false-positive rate with hundreds of negative breast biopsy results among the 4,738 women who underwent MRI screening.
MRI = magnetic resonance imaging.
The overall mortality rate in U.S. women with breast cancer is about 20 per 100,000. Mortality rates are highest in women 85 years and older (170 per 100,000). 2 White women have the highest rate of breast cancer diagnosis, whereas Black women have the highest rate of breast cancer–related death. 2 Breast cancer is also the most common cause of cancer-related death in Hispanic women and the second leading cause of cancer-related death behind lung cancer among all women. 2
Cancer screening recommendations are determined by the patient's current anatomy. Transgender females with breast tissue and transgender males who have not undergone complete mastectomy should receive screening mammography based on guidelines for cisgender persons (see https://www.aafp.org/afp/2018/1201/p645.html#sec-4 ).
What Are the Risk Factors for Breast Cancer?
The strongest risk factors are a history of childhood chest radiation, older age, increased breast density, family history of breast cancer, and certain genetic mutations ( Table 1 ). 3 – 16 However, most women who develop invasive breast cancer do not have any of these risk factors . 3
EVIDENCE SUMMARY
A retrospective cohort study demonstrated a standardized incidence ratio (i.e., the ratio of observed to expected number of cases) of 21.9 for breast cancer in women who received chest radiation during childhood. 4 Higher doses of radiation were associated with higher risk, and the highest risk was in those who received whole lung radiation (standardized incidence ratio = 43.6). The overall cumulative risk of developing breast cancer by 50 years of age was 30%. 4
Increasing age is another strong risk factor. Invasive breast cancer will be diagnosed in one out of 42 women 50 to 59 years of age, and this rate increases to one out of 14 in women 70 years and older. 5
Breast density is the amount of glandular and stromal tissue compared with adipose tissue shown on a mammogram. A systematic review and meta-analysis found that compared with women who do not have dense breasts, the relative risk of developing breast cancer is 1.79 for women with breast density between 5% and 24% and 4.64 for those with breast density of 75% or higher. 6
Data from the Breast Cancer Surveillance Consortium and the Collaborative Breast Cancer Study showed that having a first-degree relative with breast cancer increases a woman's personal risk by a hazard ratio of 1.61 and odds ratio of 1.64. 7 For patients with BRCA mutations, the risk of developing breast cancer by 80 years of age is 60% to 63%, regardless of family history. 8
How Can Physicians Estimate the Risk of Developing Breast Cancer?
Several validated risk assessment tools are available to stratify breast cancer risk ( Table 2 ). 17 These tools can assist physicians and patients in developing individualized plans regarding screening, genetic testing, or chemoprevention .
A large retrospective cohort study compared the six-year accuracy of five validated risk assessment tools among 35,921 women 40 to 84 years of age who underwent screening mammography in the United States from 2007 to 2009. 17 The models were BRCAPRO ( https://projects.iq.harvard.edu/bayesmendel/bayesmendel-r-package ); Breast Cancer Risk Assessment Tool, or Gail model ( https://bcrisktool.cancer.gov , https://www.mdcalc.com/gail-model-breast-cancer-risk ); Tyrer-Cuzick model, or International Breast Cancer Intervention Study model ( http://www.ems-trials.org/riskevaluator ); Breast Cancer Surveillance Consortium model ( https://tools.bcsc-scc.org/BC5yearRisk/calculator.htm ); and Claus model (computer program).
Based on overall performance, the positive predictive values were 2.6% for BRCAPRO and the Tyrer-Cuzick model, 2.9% for the Breast Cancer Risk Assessment Tool and Breast Cancer Surveillance Consortium model, and 3.9% for the Claus model. The negative predictive values were high at 97% or more for all of the models. 17
Does Screening Mammography Reduce Breast Cancer–Related Mortality?
Screening mammography reduces breast cancer–related mortality, with larger reductions as women get older .
Modeling studies estimate that in women 40 to 49 years of age, the number needed to screen (NNS) with annual mammography to prevent one breast cancer death is 746. The NNS decreases to 351 in women 50 to 59 years and to 233 in women 60 to 69 years. The NNS is 377 in women 70 to 79 years of age. 18 However, randomized controlled trials have demonstrated a substantially higher NNS. A meta-analysis performed for the U.S. Preventive Services Task Force (USPSTF) calculated that per 10,000 women screened with mammography, only three breast cancer deaths are avoided over 10 years in women 40 to 49 years of age, eight deaths are avoided in women 50 to 59 years, 21 deaths are avoided in women 60 to 69 years, and 13 deaths are avoided in women 70 to 74 years. 19 [ corrected ]
Between 2008 and 2017, yearly rates of newly diagnosed breast cancer increased by 0.3%, and rates of breast cancer death fell by 1.5%. 20 This may be partly attributable to early detection of small, curable breast cancers that have a five-year relative survival rate of 98.8% posttreatment. 20 Studies have shown a reduction in the incidence of large tumors, which is also likely because of early detection of smaller tumors by mammography. 21
Lower death rates, however, may also reflect improved treatments. With older treatments, the reduction in mortality after screening mammography was approximately 12 deaths per 100,000 women. With improved treatments, the reduction in mortality after screening mammography is now about eight deaths per 100,000 women. 21
What Are the Potential Harms of Breast Cancer Screening?
False-positive results are common with screening mammography, especially in younger women, leading to further imaging and radiation exposure and subsequent breast biopsies that can be painful, can cause anxiety, and usually yield benign results. Furthermore, screening can lead to overdiagnosis and overtreatment of cancers that may never have become symptomatic or life-threatening .
According to the USPSTF, the false-positive rate of mammography is highest in women 40 to 49 years of age at 121 per 1,000 and decreases with age to 69.6 per 1,000 women 70 to 79 years of age. 22 About one of every eight women 40 to 49 years of age who has a screening mammogram will subsequently undergo additional imaging, and for every case of invasive breast cancer detected by screening mammography in this age group, 10 women will have had a biopsy, compared with only three women in their 70s. 22
False-positive results are associated with increased antidepressant and anxiolytic prescriptions, with a relative risk of 1.13 to 1.19. 23 Women at highest risk of needing antidepressant and anxiolytic therapy are those 40 to 49 years of age who underwent multiple tests, including a biopsy, and who had to wait more than one week to be told the results were false-positive. 23
Systematic reviews have found that screening mammography leads to an overdiagnosis rate of 10% to 30%. 24 , 26 [ corrected ] Overdiagnosis can lead to unnecessary treatments for screening-detected breast cancers. Sometimes this involves treating ductal carcinoma in situ that would have been inconsequential over a woman's lifetime. 3 A study based on a large U.S. cancer registry reported that out of 297,000 women 40 years and older who had a mastectomy in 2013, 18% may not have needed one. 25 Thus, the USPSTF concludes that there is no strong evidence supporting mammography screening of average-risk women in their 40s. 26
What Are the Screening Recommendations for Patients at Average Risk?
Recommendations for breast self-examinations, clinical breast examinations, and mammography vary among organizations . Table 3 summarizes recommendations from the USPSTF, the American Academy of Family Physicians (AAFP), the American College of Obstetricians and Gynecologists (ACOG), the American College of Radiology (ACR), the American Cancer Society (ACS), and the National Comprehensive Cancer Network (NCCN) . 3 , 26 – 33
Breast Self-Examination . The USPSTF and AAFP recommend against teaching patients to perform breast self-examinations because of a lack of supporting evidence. 26 , 27 ACOG, the NCCN, and the ACS encourage breast self-awareness (i.e., patient familiarity with how her breasts usually feel and look) and advise women to seek medical attention if they notice breast changes. 3 , 31 , 33 There may be some rationale for breast self-awareness based on the frequency of self-detection cited in some studies. For example, out of 361 breast cancer survivors who participated in the 2003 National Health Interview Survey, 43% reported detecting their own cancers. 34
Clinical Breast Examination . The USPSTF and AAFP state that there is insufficient evidence to assess the benefits and harms of clinical breast examinations. 26 , 28 The ACS recommends against these examinations because of insufficient evidence of benefit and a high rate of false-positive results (55 false-positives for every breast cancer detected). 31 , 35 For average-risk women 40 years and older, ACOG says that annual clinical breast examinations may be offered, and the NCCN recommends annual clinical breast examinations. 3 , 33
Mammography . Evidence of benefit varies with a woman's age. The USPSTF found lower mortality rates and a reduced risk of advanced breast cancer in women 50 years and older who had mammography screening (relative risk = 0.62; 95% CI, 0.46 to 0.83) but not in women 39 to 49 years of age (relative risk = 0.98; 95% CI, 0.74 to 1.37). 19 The number of breast cancer deaths prevented with screening over 10 years was 12.5 per 10,000 women 50 years and older but only 2.9 per 10,000 women in their 40s. 19 Overall, women 50 to 59 years of age have the best balance of risks and benefits from mammography. 3 , 19
ACS data, however, showed improved mortality benefit across all age groups, although the benefit was lower in younger women. The NNS to reduce mortality rates by 20% was 1,770 for women in their 40s, 1,087 for women in their 50s, and 835 for women in their 60s. 31
The USPSTF recommends biennial screening mammography for women 50 to 74 years of age. 26 This recommendation excludes women 40 to 49 years of age because the number needed to invite (NNI) of 1,904 and the NNS of 1,034 to detect one case of breast cancer with screening mammography were considered too high. The NNI of 1,339 and NNS of 455 in women 50 to 59 years of age and the NNI of 377 and NNS of 233 for women 60 to 69 years of age were considered acceptable. 18 The AAFP supports the USPSTF recommendation. 29
The ACS recommends annual screening mammography starting at 45 years of age and transitioning to biennial screening at 55 years of age. 31 This recommendation is based on multivariable analyses suggesting that women in the younger age group are more likely than older women to have advanced stage cancer when screened biennially rather than annually. 31
The NCCN recommends annual screening mammography. 33 , 36 ACOG recommends shared decision-making based on a discussion of benefits and harms when deciding between annual and biennial screening intervals. 3
At What Age Should Breast Cancer Screening Be Discontinued?
Women at average risk should continue screening mammography through 74 years of age . 3 , 26 , 29 – 31 , 33 Starting at 75 years of age, women should be involved in shared decision-making based on overall health status and life expectancy according to ACOG recommendations . 3 The ACS and NCCN recommend continued screening after 75 years of age if life expectancy is at least 10 years, and the ACR recommends continued screening if life expectancy is at least five to seven years . 30 , 31 , 33 The USPSTF states that there is insufficient evidence to assess the benefits and harms of screening past 74 years of age, and the AAFP supports this finding . 26 , 29
Randomized controlled trials have shown that when mammography screening prevents a death, the death would have occurred within five to seven years after screening; thus, screening women with limited life expectancy is not warranted. 36 In addition, the number of life-years gained from screening decreases from 7.8 to 11.4 per 1,000 mammograms at 74 years of age to 4.8 to 7.8 per 1,000 at 80 years and to 1.4 to 2.4 per 1,000 at 90 years. 37 When adjusted for quality of life, the number of life-years gained decreases even further, and by 90 to 92 years of age, all life-years gained are counter-balanced by a loss in quality of life, presumably because of treatment adverse effects. 37 Yet, despite these data and the corresponding recommendations, 62% of women 75 to 79 years of age and 50% of women 80 years or older get mammograms, and 70% to 86% of physicians recommend mammography for 80-year-old women. 38 , 39
What Are the Screening Recommendations for Patients at Increased Risk?
ACS recommends that women with a 20% or higher lifetime risk of breast cancer (assessed using a risk assessment tool [ Table 2 17 ] ) be offered annual mammography and magnetic resonance imaging (MRI), typically starting at 30 years of age . 32 For high-risk women 25 to 29 years of age, ACOG recommends a clinical breast examination every six to 12 months and annual breast MRI with contrast. For patients 30 years and older, ACOG recommends annual mammography and MRI with contrast . 40 The NCCN recommends that women with a lifetime risk of more than 20% have breast self-awareness and receive a clinical breast examination every six to 12 months starting at 21 years of age. Annual breast MRI is recommended starting at 25 years of age with annual screening mammography starting at 30 years . 33 Women younger than 25 years with a history of chest radiation should have breast self-awareness and receive a clinical breast examination every six to 12 months starting 10 years after radiation therapy. Once these women are 25 years old, annual breast MRI is recommended, then screening mammography starting at 30 years of age . 33 The USPSTF states that there is insufficient evidence to assess the benefits and harms of using MRI for breast cancer screening, and the AAFP supports this finding . 26 , 29
The evidence for adding annual MRI screening to mammography and clinical breast examinations in women with more than a 20% lifetime risk of breast cancer is based on nonrandomized screening trials and observational studies from the 1990s. 32 These studies showed that MRI has a sensitivity of 71% to 100% for detecting breast cancer in high-risk women vs. mammography's sensitivity of 16% to 40% in the same population. However, MRI is less specific (81% to 99%) compared with mammography (93% to more than 99%), resulting in higher rates of false-positives, subsequent medical appointments, and biopsies, with a positive predictive value of 20% to 40%. No data were collected on survival rates with MRI screening or on the optimal MRI screening interval. 32
Does Supplemental Imaging Have a Role in Evaluating Dense Breasts?
Almost 50% of women 40 to 74 years of age have dense breasts, which is a risk factor for breast cancer and for false-negative results on standard mammography . 41 Ultrasonography, MRI, and digital breast tomosynthesis (also known as 3D mammography) have been proposed as methods to detect breast cancers that might be missed on mammography in women with dense breasts .
The ACR recommends considering ultrasonography in addition to screening mammography based on a randomized multicenter trial showing improved cancer detection rates compared with mammography alone (1.9 vs. 4.2 per 1,000). 30 , 42 Ultrasonography may be particularly useful for women who have a 15% to 20% lifetime risk of breast cancer and dense breasts but no additional risk factors. 43
Data from the Connecticut Experiments showed an additional 2.3 cancers detected per 1,000 women with dense breasts who were screened with ultrasonography in addition to mammography. 43 By the fourth year of the study, the positive predictive value had increased from 7.3% to 20.1%, indicating an improved learning curve for the radiologists regarding which lesions to biopsy. Another study, involving 2,662 women with dense breasts plus one other risk factor for breast cancer, showed that adding ultrasonography to mammography increased the sensitivity of breast cancer detection compared with mammography alone (52% vs. 76%). 42
It is important to note, however, that the increased sensitivity comes at the cost of increasing false-positives. An observational cohort study of 6,081 women with varying risk of breast cancer showed that the false-positive rate was 22.2 per 1,000 screens for mammography alone vs. 52 per 1,000 screens for mammography plus ultrasonography (relative risk = 2.23). 44
MRI has also been studied as a screening option in women with dense breasts. A large multicenter trial randomized women with dense breasts and a negative result on standard mammography to two-year screening with either MRI or standard mammography. 45 The cancer detection rate during the two years was lower in the MRI group than in the mammography group (2.5 vs. 5 per 1,000 screens). More than 90% of MRI-detected cancers, however, were stage 0 or 1, and MRI screening resulted in a high false-positive rate (79.8 per 1,000 screens) with hundreds of negative breast biopsy results among the 4,738 women who underwent MRI screening.
MRI has also been compared with digital breast tomosynthesis. There were higher rates of cancer detection with MRI (11.8 per 1,000 screens) than with digital breast tomosynthesis (4.8 per 1,000 screens), but no data are available on long-term outcomes. 46 A study comparing standard mammography with digital breast tomosynthesis is underway. 47
The long-term survival of women whose breast cancers were detected with supplemental imaging modalities has not been studied.
This article updates previous articles on this topic by Tirona , 48 Knutson and Steiner , 49 and Apantaku . 50
Data Sources: A PubMed search was completed in Clinical Queries using the key terms breast cancer, breast cancer screening, risk factors for breast cancer, breast cancer risk assessment tools, breast cancer screening recommendations, breast density, mammography, supplemental screening. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Also searched were the Agency for Healthcare Research and Quality Effective Healthcare Reports, the Cochrane database, and Essential Evidence Plus. Search date: April 2020.
American Cancer Society. Cancer facts and figures 2020. Accessed April 4, 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2020/cancer-facts-and-figures-2020.pdf
Centers for Disease Control and Prevention. United States cancer statistics: data visualizations. Accessed August 2, 2020. https://gis.cdc.gov/Cancer/USCS/DataViz.html
American College of Obstetricians and Gynecologists. Practice bulletin no. 179. Breast cancer risk assessment and screening in average-risk women. Obstet Gynecol. 2017;130(1):e1-e16.
Moskowitz CS, Chou JF, Wolden SL, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32(21):2217-2223.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30.
McCormack VA, dos Santos Silva I. Breast density and parenchymal patterns as markers of breast cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1159-1169.
Shiyanbola OO, Arao RF, Miglioretti DL, et al. Emerging trends in family history of breast cancer and associated risk. Cancer Epidemiol Biomarkers Prev. 2017;26(12):1753-1760.
Metcalfe KA, Lubinski J, Gronwald J, et al.; Hereditary Breast Cancer Clinical Study Group. The risk of breast cancer in BRCA1 and BRCA2 mutation carriers without a first-degree relative with breast cancer. Clin Genet. 2018;93(5):1063-1068.
Chen S, Iversen ES, Friebel T, et al. Characterization of BRCA1 and BRCA2 mutations in a large United States sample. J Clin Oncol. 2006;24(6):863-871.
American Cancer Society. Breast cancer facts and figures 2019–2020. Accessed August 2, 2020. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf
Schacht DV, Yamaguchi K, Lai J, et al. Importance of a personal history of breast cancer as a risk factor for the development of subsequent breast cancer. AJR Am J Roentgenol. 2014;202(2):289-292.
Dyrstad SW, Yan Y, Fowler AM, et al. Breast cancer risk associated with benign breast disease. Breast Cancer Res Treat. 2015;149(3):569-575.
Liu Y, Colditz GA, Rosner B, et al. Alcohol intake between menarche and first pregnancy. J Natl Cancer Inst. 2013;105(20):1571-1578.
Collaborative Group on Hormonal Factors in Breast Cancer. Type and timing of menopausal hormone therapy and breast cancer risk: individual participant meta-analysis of the worldwide epidemiological evidence. Lancet. 2019;394(10204):1159-1168.
Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk. Lancet Oncol. 2012;13(11):1141-1151.
Colditz GA, Rosner B. Cumulative risk of breast cancer to age 70 years according to risk factor status. Am J Epidemiol. 2000;152(10):950-964.
McCarthy AM, Guan Z, Welch M, et al. Performance of breast cancer risk-assessment models in a large mammography cohort. J Natl Cancer Inst. 2020;112(5):489-497.
Hendrick RE, Helvie MA. Mammography screening. AJR Am J Roentgenol. 2012;198(3):723-728.
Nelson HD, Fu R, Cantor A, et al. Effectiveness of breast cancer screening: systematic review and meta-analysis to update the 2009 U.S. Preventive Services Task Force recommendation. Ann Intern Med. 2016;164(4):244-255.
National Cancer Institute. Female breast cancer. Accessed August 2, 2020. https://seer.cancer.gov/statfacts/html/breast.html
Welch HG, Prorok PC, O'Malley AJ, et al. Breast-cancer tumor size, overdiagnosis, and mammography screening effectiveness. N Engl J Med. 2016;375(15):1438-1447.
Nelson HD, O'Meara ES, Kerlikowske K, et al. Factors associated with rates of false-positive and false-negative results from digital mammography screening. Ann Intern Med. 2016;164(4):226-235.
Segel JE, Balkrishnan R, Hirth RA. The effect of false-positive mammograms on antidepressant and anxiolytic initiation. Med Care. 2017;55(8):752-758.
Monticciolo DL, Helvie MA, Hendrick RE. Current issues in the overdiagnosis and overtreatment of breast cancer. AJR Am J Roentgenol. 2018;210(2):285-291.
Harding C, Pompei F, Burmistrov D, et al. Use of mastectomy for overdiagnosed breast cancer in the United States. J Cancer Epidemiol. ;2019:5072506.
U.S. Preventive Services Task Force. Breast cancer: screening. January 11, 2016. Accessed July 20, 2019. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/breast-cancer-screening
American Academy of Family Physicians. Breast cancer, breast self exam (BSE). Accessed July 20, 2019. https://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer-self-bse.html
American Academy of Family Physicians. Breast cancer, clinical breast examination (CBE). Accessed July 20, 2019. https://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer-cbe.html
American Academy of Family Physicians. Breast cancer. Accessed May 31, 2020. https://www.aafp.org/patient-care/clinical-recommendations/all/breast-cancer.html
Lee CH, Dershaw DD, Kopans D, et al. Breast cancer screening with imaging: recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J Am Coll Radiol. 2010;7(1):18-27.
Oeffinger KC, Fontham ETH, Etzioni R, et al.; American Cancer Society. Breast cancer screening for women at average risk [published correction appears in JAMA . 2016;315(13):1406]. JAMA. 2015;314(15):1599-1614.
Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography [published correction appears in CA Cancer J Clin . 2007;57(3):185]. CA Cancer J Clin. 2007;57(2):75-89.
Bevers TB, Helvie M, Bonaccio E, et al. NCCN clinical practice guidelines in oncology. Breast cancer screening and diagnosis. Version 3.2019. May 17, 2019. Accessed May 31, 2019. https://www.nccn.org/professionals/physician_gls/pdf/breast-screening.pdf
Roth MY, Elmore JG, Yi-Frazier JP, et al. Self-detection remains a key method of breast cancer detection for U.S. women. J Womens Health (Larchmt). 2011;20(8):1135-1139.
Meyers ER, Moorman P, Gierisch JM, et al. Benefits and harms of breast cancer screening: a systematic review [published correction appears in JAMA . 2016;315(13):1406]. JAMA. 2015;314(15):1615-1634.
Helvie MA, Bevers TB. Screening mammography for average-risk women. J Natl Compr Canc Netw. 2018;16(11):1398-1404.
van Ravesteyn NT, Stout NK, Schechter CB, et al. Benefits and harms of mammography screening after age 74 years. J Natl Cancer Inst. 2015;107(7):djv103.
Bellizzi KM, Breslau ES, Burness A, et al. Prevalence of cancer screening in older, racially diverse adults. Arch Intern Med. 2011;171(22):2031-2037.
Leach CR, Klabunde CN, Alfano CM, et al. Physician over-recommendation of mammography for terminally ill women. Cancer. 2012;118(1):27-37.
American College of Obstetricians and Gynecologists. Hereditary breast and ovarian cancer syndrome. Practice bulletin no. 182. September 2017. Accessed August 2, 2020. https://bit.ly/37Kl2M4
Sprague BL, Gangnon RE, Burt V, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014;106(10):dju255.
Berg WA, Zhang Z, Lehrer D, et al.; ACRIN 6666 Investigators. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307(13):1394-1404.
Thigpen D, Kappler A, Brem R. The role of ultrasound in screening dense breasts. Diagnostics (Basel). 2018;8(1):20.
Lee JM, Arao RF, Sprague BL, et al. Performance of screening ultrasonography as an adjunct to screening mammography in women across the spectrum of breast cancer risk [published correction appears in JAMA Intern Med . 2019;179(5):733]. JAMA Intern Med. 2019;179(5):658-667.
Bakker MF, de Lange SV, Pijnappel RM, et al.; DENSE Trial Study Group. Supplemental MRI screening for women with extremely dense breast tissue. N Engl J Med. 2019;381(22):2091-2102.
Comstock CE, Gatsonis C, Newstead GM, et al. Comparison of abbreviated breast MRI vs digital breast tomosynthesis for breast cancer detection among women with dense breasts undergoing screening [published correction appears in JAMA . 2020;323(12):1194]. JAMA. 2020;323(8):746-756.
National Cancer Institute. TMIST (Tomosynthesis Mammographic Imaging Screening Trial). Accessed January 10, 2020. https://www.cancer.gov/about-cancer/treatment/clinical-trials/nci-supported/tmist
Tirona MT. Breast cancer screening update. Am Fam Physician. 2013;87(4):274-278. Accessed September 15, 2020. https://www.aafp.org/afp/2013/0215/p274.html
Knutson D, Steiner E. Screening for breast cancer. Am Fam Physician. 2007;75(11):1660-1666. Accessed September 15, 2020. https://www.aafp.org/afp/2007/0601/p1660.html
Apantaku LM. Breast cancer diagnosis and screening. Am Fam Physician. 2000;62(3):596-602. Accessed September 15, 2020. https://aafp.org/afp/2000/0801/p596.html
Continue Reading

More in AFP
More in pubmed.
Copyright © 2021 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP. See permissions for copyright questions and/or permission requests.
Copyright © 2024 American Academy of Family Physicians. All Rights Reserved.
For the best browsing experience please enable JavaScript. Instructions for Microsoft Edge and Internet Explorer , other browsers

- About cancer
- Get involved
- Our research
- Funding for researchers
- Cancer types
- Cancer in general
- Causes of cancer
- Coping with cancer
- Health professionals
- Do your own fundraising
- By cancer type
- By cancer subject
- Our funding schemes
- Applying for funding
- Managing your research grant
- How we deliver our research
- Find a shop
- Shop online
- Our eBay shop
- Our organisation
- Current jobs
- Cancer news

Breast screening
Screening aims to find breast cancers early, when they have the best chance of being successfully treated.
- To have screening you have an x-ray of your breast called a mammogram.
- Breast screening is for women between the ages of 50 and 70, it is also for some trans or non-binary people.
- You should get a letter with your results within 2 to 3 weeks. This will tell you what you need to do next.
- There are potential benefits and risks.
Contact your breast screening service if you haven’t received your invitation or breast screening test results.
What is breast screening?
Cancer screening involves testing apparently healthy people for early signs of cancer.
Breast screening uses a test called mammography which involves taking x-rays of the breasts. Screening can help to find breast cancers early when they are too small to see or feel. These cancers are usually easier to treat than larger ones.
It is important to remember that screening will not prevent you from getting breast cancer but aims to find early breast cancers.
Overall, the breast screening programme finds cancer in around 9 out of every 1,000 women having screening.
Who has breast screening?
The NHS Breast Screening Programme invites all women from the age of 50 to 70 registered with a GP for screening every 3 years. This means that some people may not have their first screening mammogram until they are 52 or 53 years.
If you are older than 70
In England, Wales and Northern Ireland you can still have screening every 3 years but you won't automatically be invited. To continue to have screening contact your GP or your local breast screening unit.
In Scotland you can continue to have breast screening after you are 71 years of age up until your 75th birthday, but you won’t automatically be invited, you have to contact your local breast screening service.
If you are younger than 50
Your risk of breast cancer is generally very low. Mammograms are more difficult to read in younger women because their breast tissue is denser. So the patterns on the mammogram don't show up as well. There is little evidence to show that regular mammograms for women below the screening age would reduce deaths from breast cancer.
Breast screening for transgender or non-binary people
Breast screening is also for some trans or non-binary people. This includes:
- trans men and non-binary people assigned female at birth who have not had an operation to remove their breasts (bilateral mastectomy)
- trans women and non-binary people assigned male at birth who have taken feminising hormones
The screening invitations you automatically receive depend on how your sex is registered with your GP. Any hormones or surgeries you’ve had will impact which screenings are relevant for you.
If you haven’t had a breast screening invitation when you think you should, or you have any further questions, speak to your GP or gender identity clinic.
- Go to information about screening for trans and non-binary people
Find your local breast screening unit
- Northern Ireland
Tests used in breast screening
Breast screening takes 2 x-rays of each breast. The x-rays are called mammograms.
You have one mammogram from above and one from the side on each breast.
- Read about mammograms and see our video
What happens if I have breast implants?
A mammogram is still the best way to detect early breast cancer, even if you have breast implants. But a small amount of the breast tissue might be hidden by the implant.
This means that it is not as easy to see all the breast tissue, and you may have more x-rays taken. This will help the doctor see as much of the breast tissue as possible.
It is useful to let the screening unit staff know that you have implants before your mammogram.
- Find out more about breast implants and breast screening
After breast screening
You should get your results within 2 to 3 weeks. The radiographer can tell you when to expect yours. Most people have a normal reading.
If you have a normal result
You will receive a letter to let you know your mammogram does not show any signs of cancer. Your next screening appointment will be in 3 years’ time. Do contact your GP or local screening unit if you haven’t received an appointment and think you are due one.
It is important to see your GP If you notice any symptoms between your screening mammograms.
If the results aren't clear
If the x-ray isn't clear enough or shows any abnormal areas, the clinic staff will call you back for more tests. You might need to have the x-rays taken again.
If you are called back
Around 4 out of 100 women (around 4%) are called back for more tests. If this happens, you might feel very worried. But many of these women won’t have cancer.
If you are called back because your mammogram showed an abnormal area, you might have a magnified mammogram. This can show up particular areas of the breasts more clearly. These mammograms show the borders of any lump or thickened area. They can also show up areas of calcium (calcification).

- Find out about tests to diagnose breast conditions
- Find out about symptoms of breast cancer
Possible benefits of breast screening
Breast cancers found by screening are generally at an early stage. Very early breast cancers are usually easier to treat, may need less treatment, and are more likely to be successfully treated.
The current evidence suggests that breast screening reduces the number of deaths from breast cancer by about 1,300 a year in the UK.
Almost all women diagnosed with breast cancer at the earliest possible stage in England survive their disease for at least 5 years after diagnosis.
- Find out more about survival for breast cancer
Risks of breast screening
Although breast screening can find many cancers early, it isn't perfect. There are some risks, and some people may have a false positive or false negative result.
What is a false negative result?
Screening doesn't always find a cancer that is there. So some people with breast cancer will be missed. This is called a false negative result.
What is a false positive result?
In some women, the test picks up something even though they don't have breast cancer. This is called a false positive result and can lead to anxiety and further tests such as a breast biopsy.
Overdiagnosis and overtreatment
As well as finding cancers that need treating, screening can also pick up breast cancers that won't ever cause any problems.
At the moment it isn't possible to know whether a breast cancer will grow quickly and need treatment, or will grow slowly, or not at all. So, almost all women diagnosed have surgery to remove the cancer. Many also have radiotherapy, hormone therapy or chemotherapy.
For some people the treatment is unnecessary but at the moment doctors can't tell who needs treatment and who doesn't.
Screening can also pick up changes in the lining of the breast ducts called ductal carcinoma in situ (DCIS). It isn't possible to tell whether DCIS will develop into a cancer or not. So, many women with DCIS also have surgery and radiotherapy or hormone therapy.
- Read about DCIS
A 2012 breast screening review found that screening leads to around 4,000 women overdiagnosed in the UK each year.
Exposure to radiation
Each mammogram exposes a person to small amounts of radiation from the x-rays. But the amount of radiation is very small.
X-rays can very rarely cause cancer. Having mammograms every 3 years for 20 years very slightly increases the chance of getting cancer over a woman’s lifetime.
The balance of benefit and harms
An NHS digital report found that more than 20,100 breast cancers were diagnosed through screening in England between 2021 and 2022.
Of those breast cancer cases detected most of these were found at an early stage.
Treatment is likely to be more successful if the cancer is an early stage.
Screening for women at higher risk
Speak to your GP if you think you might be at an increased risk. They can refer you to a genetic specialist, who can assess your risk. Not everyone with a family history of cancer is at increased risk themselves.
If you have a family history of breast cancer
UK guidelines recommend that women with a moderate or high risk of breast cancer because of their family history should start having screening mammograms every year in their forties.
If you have a gene mutation
If you have had tests that showed a mutation that increases the risk of breast cancer, the recommendations are slightly different.
UK guidelines recommend yearly MRI scans from:
- age 20 for women with a TP53 mutation
- Read about having family members with breast cancer
Should I go for breast screening?
It’s important that you have access to enough information about the benefits and harms of breast screening to make the decision.
You can talk to your own doctor or nurse. Or you can contact the Cancer Research UK information nurses on freephone 0808 800 4040. The lines are open from 9am to 5pm, Monday to Friday.
- Find out more information about breast screening
Breast awareness
Even if you are having mammograms every 3 years it is important to make sure that you know how your breasts normally look and feel. Cancers can develop between mammograms. This is known as an interval cancer. Mammograms can also miss some cancers.
If you notice any unusual changes in your breast don’t wait until your next mammogram. See your GP straight away.
- Read about finding breast cancers early
Information for people with learning disabilities
You can watch a video about women with learning disabilities who are going to have breast screening. The video was produced by Avon Breast Screening. It is about 11 minutes long.
- Watch the Avon Breast Screening video for women with learning disabilities
For people with a learning disability NHS England have an easy read leaflet about breast screening as well as links to other resources for those that cannot read.
- Go to NHS England easy read guide about breast screening
British Sign Language (BSL) information
There is BSL information about breast screening in the different UK nations. Public Health Wales have 3 BSL videos about breast screening. They are about 35 minutes, 9 minutes and 7 minutes long.
- Go to the Public Health Wales BSL videos
NHS information have 2 videos about breast screening in Scotland. These are about 9 minutes and 11 minutes long.
- Watch the NHS inform BSL videos about breast screening
The Public Health Agency in Northern Ireland have a British sign language and Irish sign language video. You can find these towards the bottom of the page and are both about 5 minutes long.
- View the HSC British sign language and Irish sign language videos (the links are at the bottom)
JoC's breast cancer story
JoC was diagnosed when she attended a screening appointment.
"I actually didn't have any idea that I had cancer."
- This is JoC's story about her diagnosis and treatment
Related links
Symptoms of breast cancer.
Symptoms of breast cancer include a lump or thickening in the breast. Find out more about this and other possible symptoms and when you should see your GP.
Tests for breast cancer
You have a number of tests to check for breast cancer. This includes a breast examination, a mammogram, a biopsy and scans.
Stages and grades of breast cancer
Get information about how doctors stage and grade breast cancer. In the UK, doctors use the TNM system to stage breast cancer. You may also be told about the number staging system.
Treatment for breast cancer
Treatment for breast cancer depends on a number of factors. Find out about breast cancer treatments, where and how you have them, and how to cope with possible side effects.
What is breast cancer?
Breast cancer is cancer that starts in the breast tissue. Find out about who gets breast cancer and where it starts.
Breast cancer main page
Find out about breast cancer, including symptoms, diagnosis, treatment, survival, and how to cope with the effects on your life and relationships.
It’s a worrying time for many people and we want to be there for you whenever - and wherever - you need us. Cancer Chat is our fully moderated forum where you can talk to others affected by cancer, share experiences, and get support. Cancer Chat is free to join and available 24 hours a day.
Visit the Cancer Chat forum
About Cancer generously supported by Dangoor Education since 2010.

Find a clinical trial
Search our clinical trials database for all cancer trials and studies recruiting in the UK
Cancer Chat forum
Talk to other people affected by cancer
Nurse helpline 0808 800 4040
Questions about cancer? Call freephone 9 to 5 Monday to Friday or email us

When should you get a mammogram? Austin doctors explain breast cancer screening guidelines
T he new breast cancer screening guidelines from the U.S. Preventive Services Task Force might be confusing if you are between ages 40 and 49. While the task force recommended screenings start at 40 instead of 50 with the option to start at 40, it recommended an every-other-year mammogram instead of an annual one. The task force is generally considered the authority on screening recommendations.
Those recommendations are different from the American College of Obstetricians and Gynecologists , which recommends annual or every-other-year screenings based on a conversation between the patient and doctor starting at age 40, and the American Cancer Society , which advocates for the option of annual screenings at age 40 but definitely annual screening beginning at 45. Then at age 55, a person can continue the annual screenings or opt for every-other-year screenings.
Recommendations on when to stop annual mammograms are even more confusing. The American Cancer Society recommends continuing mammograms as long as a person is healthy and expected to live another 10 years or more. The task force is asking for more data and research into this question. The doctor's association recommends screenings through age 75 and then a shared decision-making process based on a patient's health and expected longevity.
We asked local doctors to help us sort out these recommendations.
What is the advantage of starting mammograms at age 40?
Starting mammograms at age 40 can be emotional. "It hits people," said Dr. Allison Devine, an obstetrician and gynecologist with Austin Diagnostic Clinic. "It's the first introduction into a discussion about perimenopause."
While ages 50-64 are the most common ages for a woman diagnosed with breast cancer, the risk of a breast cancer diagnosis begins to increase at age 40. Moving the guidelines to age 40 can increase the survival rate by 19%, the task force cited.
"If you start at age 50, you're preventing a lot of women from getting the screening they need and finding early breast cancer," said Dr. Brett Deatherage, a radiologist at Ascension Texas Imaging in Kyle.
Cancer caught in an earlier stage means a less invasive surgery, less recovery time and the possibility of not needing additional treatment like chemotherapy or radiation.
A lot of the breast cancer found in people ages 40 to 49 is in the beginning stages. Other breast cancers found in this age group might be more aggressive and fast-growing.
Devine has had no issues getting insurance to cover an annual mammogram for women in their 40s.
Should I do a mammogram every year or every other year?
Devine follows the association's guidelines and has a conversation with her patients, but she does encourage getting mammograms every year beginning at age 40.
"I've seen plenty of women go from a completely normal mammogram without any need for follow-up testing and then the next year, they have breast cancer," Devine said.
Getting on an annual schedule also makes people more likely to get a mammogram every year rather than forgetting if this was their year or not, she said.
Dr. Julie Sprunt, a breast surgeon with Texas Breast Specialists, also encourages annual mammograms. "If we look at breasts every year, we find things earlier," she said.
That could be calcifications, which could become cancer, or a cancer being found when it is still contained or when it is ductal carcinoma in situ. Typically, treatment for ductal carcinoma in situ and breast cancer that has not spread is just a surgical removal of the cancer with no chemotherapy and no lymph nodes needing to be removed, Sprunt said.
Earlier and more frequent mammography gives radiologists like Deatherage more past mammograms to compare against the current mammograms. Using 3D mammography with artificial intelligence called computer assisted detection can help alert radiologists of suspicious areas they might not see and can help spot changes from previous mammograms.
Read more: Will you get breast cancer in five years? Austin-based Clairity working on predictive tech
Are there any disadvantages to starting mammograms at age 40?
In this age range, it's more common to get a call back for an additional screening and then find nothing to worry about. Those call backs, in which an additional mammogram is done as well as an ultrasound, can be incredibly stressful.
More women in their 40s have dense breast tissue, which can increase the call-back rates. Having a 3D mammogram instead of a 2D one can help reduce the number of call backs because it allows the radiologist to better see a tumor through the dense breasts.
New FDA guidelines require mammogram reports to let patients know whether they have a dense breast pattern.
People who are pregnant in their 40s should skip the mammogram while pregnant. If they know they are going to get pregnant, they can do one before the pregnancy begins.
Are there people who should start mammograms earlier?
The guidelines are based on the average risk for women, Devine said. "A lot of things can increase a person's risk." That includes family history, genetics and ethnicity. Women of Eastern European Jewish descent and African Americans have an increased risk.
The conversation about breast cancer risk should begin when a woman is in her late 20s or early 30s, if not before, Devine said.
Anyone who has a family history of breast cancer should begin screening mammograms 10 years before that family member was diagnosed. So, if someone has an aunt, mother, sister or grandmother who had breast cancer diagnosed at age 45, they need to be screened at age 35.
Read more: Writing back to cancer: One woman's messages inspire following
What happens if I get a call back for additional screening?
Getting called back does not mean you have cancer, Deatherage said. It just means there is something on your mammogram that the radiologist wasn't sure about or they didn't get enough imaging. "It most cases, it will not be a breast cancer," he said.
What can I do to make a mammogram less painful?
The squish isn't fun. Devine encourages people who've had a bad experience to go somewhere else. "A lot depends on the tech," she said.
Also a mammogram can be more painful just before or during menstruation.
If someone really doesn't want a mammogram, Devine might order an ultrasound, which can find a mass, but not the calcifications of early breast cancer. "Some screening is better than no screening," she said.
What else should I do other than get a mammogram?
Of course, anyone with breasts can get breast cancer, including men, who don't have screening guidelines.
Like many cancers, breast cancer rates increase with other health factor risks like obesity and alcohol use. Regular exercise, along with a diet with lean protein, fruits and vegetables that's low on processed foods can help. Breast feeding also reduces the risk.
While the vast majority of people in whom Deatherage finds a suspected breast cancer on their mammogram are older than 40, "Sometimes I see women in their 30s that have no family history," he said.
Beginning at age 20, women should do monthly breast checks, preferably on the same time of the month after your menstrual cycle. Do that screening both standing up and sitting down. Breast checks help you know what your breasts feel like normally, making it more likely you'll feel when something has changed, Sprunt said.
"Many women in their 40s pick up their own breast cancer in the interim between mammograms," Devine said.
This article originally appeared on Austin American-Statesman: When should you get a mammogram? Austin doctors explain breast cancer screening guidelines

Breast Cancer Screening (PDQ®)–Patient Version
What is screening.
Screening is looking for signs of disease, such as breast cancer , before a person has symptoms . The goal of screening tests is to find cancer at an early stage when it can be treated and may be cured . Sometimes a screening test finds cancer that is very small or very slow growing. These cancers are unlikely to cause death or illness during the person's lifetime.
Scientists are trying to better understand which people are more likely to get certain types of cancer. For example, they look at the person's age, their family history , and certain exposures during their lifetime. This information helps doctors recommend who should be screened for cancer, which screening tests should be used, and how often the tests should be done.
It is important to remember that your doctor does not necessarily think you have cancer if he or she suggests a screening test. Screening tests are done when you have no cancer symptoms. Women who have a strong family history or a personal history of cancer or other risk factors may also be offered genetic testing .
If a screening test result is abnormal , you may need to have more tests done to find out if you have cancer. These are called diagnostic tests , rather than screening tests.
For more information about cancer screening, see Cancer Screening Overview .
General Information About Breast Cancer
Breast cancer is a disease in which malignant (cancer) cells form in the tissues of the breast., breast cancer is the second leading cause of death from cancer in american women., different factors increase or decrease the risk of breast cancer..
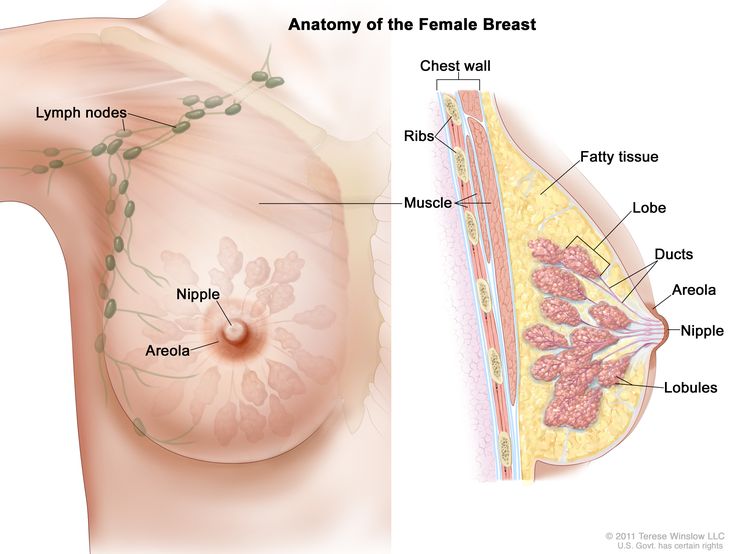
Each breast also has blood vessels and lymph vessels . The lymph vessels carry an almost colorless, watery fluid called lymph . Lymph vessels carry lymph between lymph nodes . Lymph nodes are small, bean-shaped structures that filter lymph and store white blood cells that help fight infection and disease. Groups of lymph nodes are found near the breast in the axilla (under the arm), above the collarbone , and in the chest.
For more information about breast cancer , see the following:
- Breast Cancer Prevention
- Breast Cancer Treatment (Adult)
- Male Breast Cancer Treatment
- Genetics of Breast and Gynecologic Cancers
Women in the United States get breast cancer more than any other type of cancer except for skin cancer .
Breast cancer is more likely to occur as a woman ages. It occurs more often in White women than in Black women, but Black women die from breast cancer more often than White women.
Breast cancer rarely occurs in men. Because men with breast cancer usually have a lump that can be felt, screening tests are not likely to be helpful.
For information about risk factors and protective factors for breast cancer, see Breast Cancer Prevention .
Breast Cancer Screening
Tests are used to screen for different types of cancer when a person does not have symptoms., mammography is the most common screening test for breast cancer., magnetic resonance imaging (mri) may be used to screen women who have a high risk of breast cancer., whether a woman should be screened for breast cancer and the screening test to use depends on certain factors., breast exam, thermography, tissue sampling, screening tests for breast cancer are being studied in clinical trials..
Scientists study screening tests to find those with the fewest harms and most benefits. Cancer screening trials also are meant to show whether early detection (finding cancer before it causes symptoms ) helps a person live longer or decreases a person’s chance of dying from the disease. For some types of cancer, the chance of recovery is better if the disease is found and treated at an early stage .
A mammogram is a picture of the inside of the breast . Mammography may find tumors that are too small to feel. It may also find ductal carcinoma in situ (DCIS). In DCIS, abnormal cells line the breast duct , and in some women may become invasive cancer .
There are different types of mammograms:
- Film mammography is an x-ray picture of the breast.
- Digital mammography (DM) is a computer picture of the breast.
- Digital breast tomosynthesis (DBT) uses x-rays to take a series of pictures of the breast from many different angles. A computer is used to make 3-D pictures of the breast from these x-rays.
- 2-dimensional mammography (S2D) uses x-rays to take pictures of the inside of the breast, usually from two different angles. A computer or x-ray film is used to make 2-D pictures of the breast.
Digital breast tomosynthesis (DBT) was approved by the U.S. Food and Drug Administration (FDA) in 2018 and is now used in 3 out of 4 facilities. One recent study found that 2-dimensional mammography (S2D) combined with DBT improved tumor detection rates and lowered mammogram callbacks, radiation dose , and overall costs. More studies are being done to compare different types of breast cancer screening.
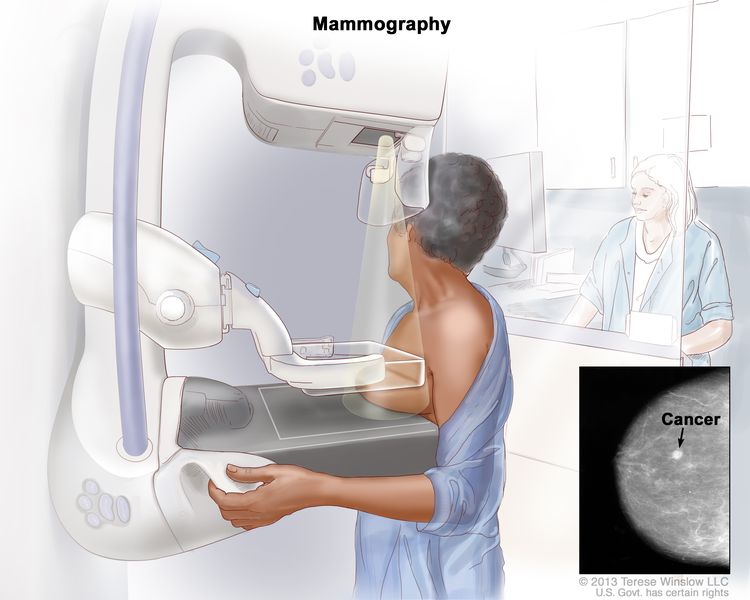
Many factors affect whether mammography is able to detect (find) breast cancer:
- The age and weight of the patient.
- The size and type of tumor.
- Where the tumor has formed in the breast.
- How sensitive the breast tissue is to hormones .
- How dense the breast tissue is.
- The timing of the mammography within the woman's menstrual cycle .
- The quality of the mammogram picture.
- The skill of the radiologist in reading the mammogram.
Women aged 50 to 69 years who have screening mammograms have a lower chance of dying from breast cancer than women who do not have screening mammograms.
Fewer women are dying of breast cancer in the United States, but it is not known whether the lower risk of dying is because the cancer was found early by screening or whether the treatments were better.
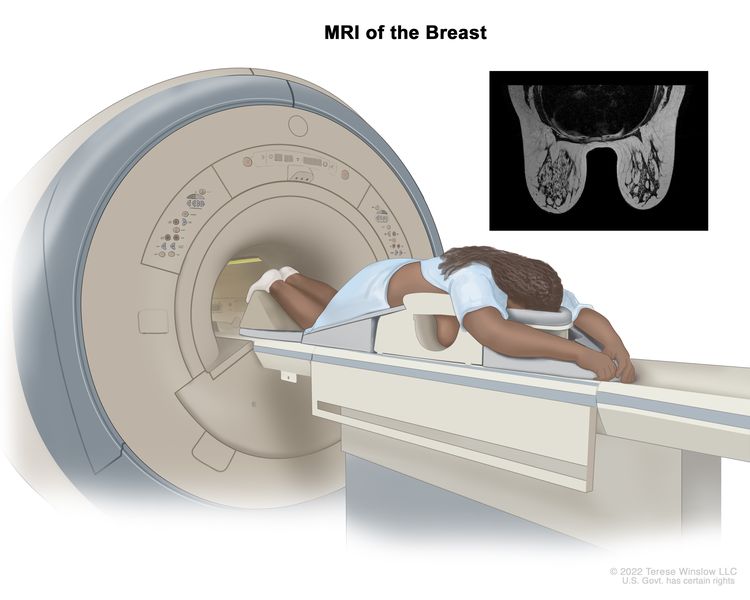
MRI may be used as a screening test for women who have a high risk of breast cancer. Factors that put women at high risk include the following:
- Certain gene changes, such as changes in the BRCA1 or BRCA2 genes.
- A family history ( first degree relative , such as a mother, daughter or sister) with breast cancer.
- Certain genetic syndromes , such as Li-Fraumeni or Cowden syndrome .
An MRI is more likely than mammography to find a breast mass that is not cancer.
Women with dense breasts who have supplemental screening (for example, an MRI) show higher rates of breast cancer detection, but there is limited evidence about whether this leads to better health outcomes.
Women with risk factors for breast cancer, such as certain changes in the BRCA1 or BRCA2 gene or certain genetic syndromes may be screened at a younger age and more often.
Women who have had radiation treatment to the chest, especially at a young age, may start routine breast cancer screening at an earlier age. The benefits and risks of mammograms and MRIs for these women have not been studied.
Breast cancer screening has not been shown to benefit the following women:
- Elderly women who, if diagnosed with breast cancer through screening, will usually die of other causes. Screening mammograms for those aged 66 to 79 years may find cancer in a very small percentage of women, but most of these cancers are low risk.
- In women with an average risk of developing breast cancer, screening mammography before age 40 has not shown any benefit.
- In women who are not expected to live for a long time and have other diseases or conditions , finding and treating early stage breast cancer may reduce their quality of life without helping them live longer.
Other screening tests have been or are being studied in clinical trials.
Studies have been done to find out if the following breast cancer screening tests are useful in finding breast cancer or helping women with breast cancer live longer.
A clinical breast exam is an exam of the breast by a doctor or other health professional. He or she will carefully feel the breasts and under the arms for lumps or anything else that seems unusual. It is not known if having clinical breast exams decreases the chance of dying from breast cancer.
Breast self-exams may be done by women or men to check their breasts for lumps or other changes. If you feel any lumps or notice any other changes in your breasts, talk to your doctor. Doing regular breast self-exams has not been shown to decrease the chance of dying from breast cancer.
Thermography is a procedure in which a special camera that senses heat is used to record the temperature of the skin that covers the breasts. Tumors can cause temperature changes that may show up on the thermogram.
There have been no randomized clinical trials of thermography to find out how well it detects breast cancer or the harms of the procedure.
Breast tissue sampling is taking cells from breast tissue to check under a microscope . Breast tissue sampling as a screening test has not been shown to decrease the risk of dying from breast cancer.
Information about clinical trials supported by NCI can be found on NCI’s clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Harms of Breast Cancer Screening
Screening tests can have harms., false-positive test results can occur., false-positive results can lead to extra testing and cause anxiety., false-negative test results can delay diagnosis and treatment., finding breast cancer may lead to breast cancer treatment and side effects, but it may not improve a woman's health or help her live longer., mammography exposes the breast to low doses of radiation., there may be pain or x-ray discomfort during a mammogram., talk to your doctor about your risk of breast cancer and your need for screening tests..
Not all breast cancers will cause death or illness in a woman's lifetime, so they may not need to be found or treated.
Decisions about screening tests can be difficult. Not all screening tests are helpful and most have harms. Before having any screening test, you may want to discuss the test with your doctor. It is important to know the harms of the test and whether it has been proven to reduce the risk of dying from cancer .
The harms of mammography include the following:
Screening test results may appear to be abnormal even though no cancer is present. A false-positive test result (one that shows there is cancer when there really isn’t) is usually followed by more tests (such as biopsy ), which also have risks.
When a breast biopsy result is abnormal, getting a second opinion from a different pathologist may confirm a correct breast cancer diagnosis .
Most abnormal test results turn out not to be cancer. False-positive results are more common in the following:
- Younger women (under age 50).
- Women who have had previous breast biopsies.
- Women with a family history of breast cancer.
- Women who take hormones for menopause .
False-positive results are more likely the first time screening mammography is done than with later screenings. For every ten women who have a single mammogram , one will have a false-positive result. The chance of having a false-positive result goes up the more mammograms a woman has. Comparing a current mammogram with a past mammogram lowers the risk of a false-positive result.
The skill of the radiologist also can affect the chance of a false-positive result.
If a mammogram is abnormal, more tests may be done to diagnose cancer. Women can become anxious during the diagnostic testing. Even if it is a false-positive test and cancer is not diagnosed, the result can lead to anxiety anywhere from a few days to years later.
Several studies show that women who feel anxiety after false-positive test results are more likely to schedule regular breast screening exams in the future.
Screening test results may appear to be normal even though breast cancer is present. This is called a false-negative test result . A woman who has a false-negative test result may delay seeking medical care even if she has symptoms . About one in 5 cancers are missed by mammography.
The chance of a false-negative test result is more common in women who:
- Are younger.
- Have dense breast tissue .
- Have cancer that is not dependent on hormones ( estrogen and progesterone ).
- Have cancer that is fast growing.
Some breast cancers found only by screening mammography may never cause health problems or become life-threatening. Finding these cancers is called overdiagnosis . When these cancers are found, having treatment may cause serious side effects and may not lead to a longer, healthier life.
Being exposed to high radiation doses is a risk factor for breast cancer. The radiation dose with a mammogram is very low. Women who start getting mammograms after age 50 have very little risk that the overall exposure to radiation from mammograms throughout their lives will cause harm. Women with large breasts or with breast implants may be exposed to slightly higher radiation doses during screening mammography.
During a mammogram, the breast is placed between two plates that are pressed together. Pressing the breast helps to get a better of the breast. Some women have pain or discomfort during a mammogram. The amount of pain may also depend on the following:
- The phase of the woman's menstrual cycle .
- The woman's anxiety level.
- How much pain the woman expected.
Talk to your doctor or other care provider about your risk of breast cancer, whether a screening test is right for you, and the benefits and harms of the screening test. You should take part in the decision about whether you want to have a screening test, based on what is best for you. For more information, see Cancer Screening Overview .
About This PDQ Summary
Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish .
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government’s center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about breast cancer screening. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Updated") is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Screening and Prevention Editorial Board .
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI's website . For more information, call the Cancer Information Service (CIS), NCI's contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary].”
The best way to cite this PDQ summary is:
PDQ® Screening and Prevention Editorial Board. PDQ Breast Cancer Screening. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/breast/patient/breast-screening-pdq . Accessed <MM/DD/YYYY>. [PMID: 26389160]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online . Visuals Online is a collection of more than 3,000 scientific images.
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s E-mail Us .
- Share full article
Advertisement
Supported by
F.D.A. Panel Endorses Safety of Colon-Cancer Blood Test
The Guardant Health Shield test, one committee member said, “is better than nothing for patients who are getting nothing, but it is not better than a colonoscopy.”

By Gina Kolata
A committee of experts that advises the Food and Drug Administration voted by large majorities on Thursday that a new blood test to screen people for colon and rectal cancers was safe and effective, and that its benefits outweighed its risks.
But the group cautioned that the blood test had limitations and added that they were endorsing it with the hope that it would increase the abysmally low number of people who are regularly screened for this cancer.
The F.D.A. usually follows the advice of its expert committees.
In the United States, about 150,000 people are diagnosed with colon and rectal cancers annually, and about 53,000 are expected to die this year. Most who are screened for the disease receive a colonoscopy or a fecal test. The F.D.A. approved these methods long ago, and research has demonstrated that they are more accurate than the new blood test, Shield, made by Guardant Health of Palo Alto, Calif.
But for people with average risk of the disease, a blood test would offer convenience — no difficult preparation, fasting or anesthesia needed as is the case for a colonoscopy, no ick factor of a self-administered fecal test. It still must be followed by a colonoscopy if cancers or pre-cancers are detected.
The biggest issue with the blood tests is that, unlike colonoscopies, they miss most of the precancerous growths on the colon that, if detected and removed, would prevent a person from developing cancer. That, said Dr. Stephen M. Hewitt, a committee member from the National Cancer Institute, “really undermines the concept of cancer prevention.”
The test, said Charity J. Morgan, a committee member who is a biostatistics professor at the University of Alabama, Birmingham, “is better than nothing for patients who are getting nothing, but it is not better than a colonoscopy.”
And there are a lot of people who are getting nothing.
The F.D.A. noted that a third of people who should be screened for colorectal cancer are not getting screened and more than 75 percent who died had not been up to date with screening.
If the agency approves the Guardant Health test, the hope is that it could fundamentally change the discouraging statistics on colon cancer by giving patients of average risk who refuse colonoscopies a convenient option to be screened.
Colon cancer is one of the only cancers that can actually be prevented with screening. That is because the disease starts slowly as a polyp, a small, harmless growth on the colon wall. Most polyps never cause any problems but a few eventually turn into cancer. If they are detected and cut out, the cancer is avoided.
Even if a polyp is missed and a cancer develops, it usually can be effectively treated if it is found before it spreads. The five-year survival rate for early colorectal cancer is 91 percent, compared with 14 percent if the cancer has metastasized.
The Guardant test found 83 percent of colorectal cancers but only 13 percent of dangerous polyps.
Colonoscopies find 95 percent of the dangerous polyps, and the most advanced fecal test finds 42 percent of them. The Guardant test is less accurate because its task is so difficult. It must find minuscule fragments of DNA from dead colon cells that end up in the blood.
The company argues that because its blood tests can be done easily and frequently, there is a higher likelihood that dangerous polyps eventually will be found in the years it takes them to develop into cancers. That, though, has yet to be demonstrated.
And the committee considered a lingering question: Is the risk that the blood test will miss a dangerous polyp balanced by the likelihood that it could greatly increase the number of people who are screened?
For some committee members the answer clearly is yes. Any screening is better than none.
“The important thing is to get more people screened in some fashion,” said Dr. Alexander D. Borowsky, a professor in the department of pathology and laboratory medicine at the U.C. Davis School of Medicine.
Gina Kolata reports on diseases and treatments, how treatments are discovered and tested, and how they affect people. More about Gina Kolata
The Fight Against Cancer
We asked experts what to know about melanoma symptoms, treatment and prevention. Here’s how to avoid one of the deadliest forms of skin cancer.
Colon and rectal cancers are increasing among people younger than 50. Experts have a few ideas about why .
Should alcoholic beverages have cancer warning labels? Ireland will require them starting in 2026, and there are nascent efforts elsewhere .
Risk calculators can offer a more personalized picture of an individual patient’s breast cancer risk. But experts warn that the results need to be interpreted with the help of a doctor .
The human papillomavirus vaccine provides powerful protection against the leading cause of cervical cancer and against a strong risk factor for anal cancer. Here’s what to know about the shot .
A recent study adds to growing evidence that exercise is an important part of preventing prostate cancer , the second most common and second most fatal cancer in the United States for men.

IMAGES
COMMENTS
Three guidelines recommended screening every 1-2 years [ [8], [23], [32] ]. Some guidelines agreed that screening intervals should be determined based on age [ 18, 24 ]. ACS [ 23] recommended screening with MAM annually for women aged 40-54 years and every 1-2 years for women aged 55 years or older.
Previous reviews of breast cancer screening effectiveness established the benefits and harms of mammography based primarily on large, long-term trials. 8,9 In 2016, the US Preventive Services Task Force (USPSTF) ... Breast cancer is an active area of research, yet few longitudinal RCTs comparing different screening strategies have been ...
MRI has been promoted as a screening test for breast cancer among women at elevated risk of breast cancer based on BRCA1/2 mutation carriers, a strong family history of breast cancer, or several genetic syndromes, such as Li-Fraumeni syndrome or Cowden disease.[3-5] Breast MRI is more sensitive but less specific than screening mammography [6,7 ...
Breast Cancer: Screening April 30, 2024. ... Breast cancer is an active area of research, yet few longitudinal RCTs comparing different screening strategies have been conducted following completion of the major trials that established the effectiveness of mammography for reducing breast cancer mortality for women aged 50 to 69 years.
Several comments suggested that there should be no upper age limit for breast cancer screening or that an upper age should be based on life expectancy. In response, the USPSTF notes that no trials of breast cancer screening enrolled women 75 years or older and an emulated trial showed no benefit to screening women aged 75 to 79 or 80 to 84.
NCI is funding a large-scale randomized breast screening trial, the Tomosynthesis Mammographic Imaging Screening Trial (TMIST), to compare the number of advanced cancers detected in women screened for 5 years with 3-D mammography with the number detected in women screened with 2-D mammography. Two concerns in breast cancer screening, as in all ...
Clinical trials are research studies that involve people. The clinical trials on this list are for breast cancer screening. All trials on the list are NCI-supported clinical trials, which are sponsored or otherwise financially supported by NCI. NCI's basic information about clinical trials explains the types and phases of trials and how they ...
Credit: BSIP/Universal Images Group/Getty. Radiologists using an artificial-intelligence (AI) assistant during breast-cancer screening had better chances of detecting cancer than those who did not ...
Screening for breast cancer: Research is needed to determine the benefits and harms of screening for breast cancer in women age 75 years or older. Research is needed to help clinicians and patients understand the best strategy for breast cancer screening in women found to have dense breasts on a screening mammogram, which occurs in more than 40 ...
Recent evidence of supplemental abbreviated MRI in women at average risk with dense breasts and negative digital tomosynthesis results appears to increase the prevalent cancer detection rate (up ...
Breast cancer screening means checking a woman's breasts for cancer before there are signs or symptoms of the disease. ... The US Preventive Services Task Force is an organization made up of doctors and disease experts who look at research on the best way to prevent diseases and make recommendations on how doctors can help patients avoid ...
The American Cancer Society (ACS) breast cancer screening guidelines consider having had either a 2D or 3D mammogram as being in line with current screening recommendations. The ACS also believes that women should be able to choose between 2D and 3D mammography if they or their doctor believes one would be more appropriate, and that out-of ...
The International Agency for Research on Cancer (IARC) has updated its 2002 guidelines on screening for breast cancer, drawing on data from studies completed in the past 15 years.
The most recent data on cancer screening rates are cause for concern, which we also noted in 2018. 20 As described above, although CRC screening rates have steadily risen, screening rates for cervical cancer have declined since 2005, breast cancer screening rates have remained stable at a discouragingly low level, and uptake of lung cancer ...
Posted: January 20, 2023. Many young women who are diagnosed with early-stage breast cancer want to become pregnant in the future. New research suggests that these women may be able to pause their hormone therapy for up to 2 years as they try to get pregnant without raising the risk of a recurrence in the short term.
Research 315; Commentary 192; Other 167; Review 81; Clinical Cases 77; Perspective 41; Media 29; ... The evidence for breast cancer screening of women in their 40s is insufficient to support a new ...
The overall cumulative risk of developing breast cancer by 50 years of age was 30%. 4. Increasing age is another strong risk factor. Invasive breast cancer will be diagnosed in one out of 42 women ...
Possible environmental causes of breast cancer have also received more attention in recent years. While much of the science on this topic is still in its earliest stages, this is an area of active research. Breast cancer prevention. Researchers are looking for ways to help reduce breast cancer risk, especially for women who are at high risk.
In fact, studies show that MRI is the best supplemental screening option for average- or intermediate-risk women with dense breasts who had a negative mammogram, with pooled data from 22 studies ...
Cancer screening involves testing apparently healthy people for early signs of cancer. Breast screening uses a test called mammography which involves taking x-rays of the breasts. Screening can help to find breast cancers early when they are too small to see or feel. ... Cancer Research UK is a registered charity in England and Wales (1089464 ...
A mammogram is an x-ray picture of the breast. Mammograms can be used to check for breast cancer in women who have no signs or symptoms of the disease. This type of mammogram is called a screening mammogram. Screening mammograms usually involve two or more x-ray pictures, or images, of each breast. The x-ray images often make it possible to ...
T he new breast cancer screening guidelines from the U.S. Preventive Services Task Force might be confusing if you are between ages 40 and 49. While the task force recommended screenings start at ...
The research published in the journal Radiology looked retrospectively at the results from almost 5,000 breast cancer screening mammograms performed at Duke University Medical Center between 2016 ...
Screening is looking for signs of disease, such as breast cancer, before a person has symptoms.The goal of screening tests is to find cancer at an early stage when it can be treated and may be cured.Sometimes a screening test finds cancer that is very small or very slow growing. These cancers are unlikely to cause death or illness during the person's lifetime.
By Gina Kolata. May 23, 2024. A committee of experts that advises the Food and Drug Administration voted by large majorities on Thursday that a new blood test to screen people for colon and rectal ...